
In which of the following species S-atom assumes $s{{p}^{3}}$-hybrid state?
(I)- $S{{O}_{3}}$
(II)- ${{H}_{2}}S$
(III)- $S{{O}_{2}}$
(IV)- ${{S}_{8}}$
(a)- (I) and (II)
(b)- (II) and (III)
(c)- (II) and (IV)
(d)- (III) and (IV)
Answer
567.3k+ views
Hint: In the compound, if the central atom should have $s{{p}^{3}}$-hybrid state, then all the bonds in the compound must be a single bond. If the compound has a double or triple bond then the central atom cannot have $s{{p}^{3}}$-hybrid state. If a single bond is not present, then the lone pair also contributes to $s{{p}^{3}}$-hybrid state.
Complete step by step solution:
In the compound, if the central atom should have $s{{p}^{3}}$-hybrid state, then all the bonds in the compound must be a single bond. If the compound has a double or triple bond then the central atom cannot have $s{{p}^{3}}$-hybrid state. If a single bond is not present, then the lone pair also contributes to $s{{p}^{3}}$-hybrid state.
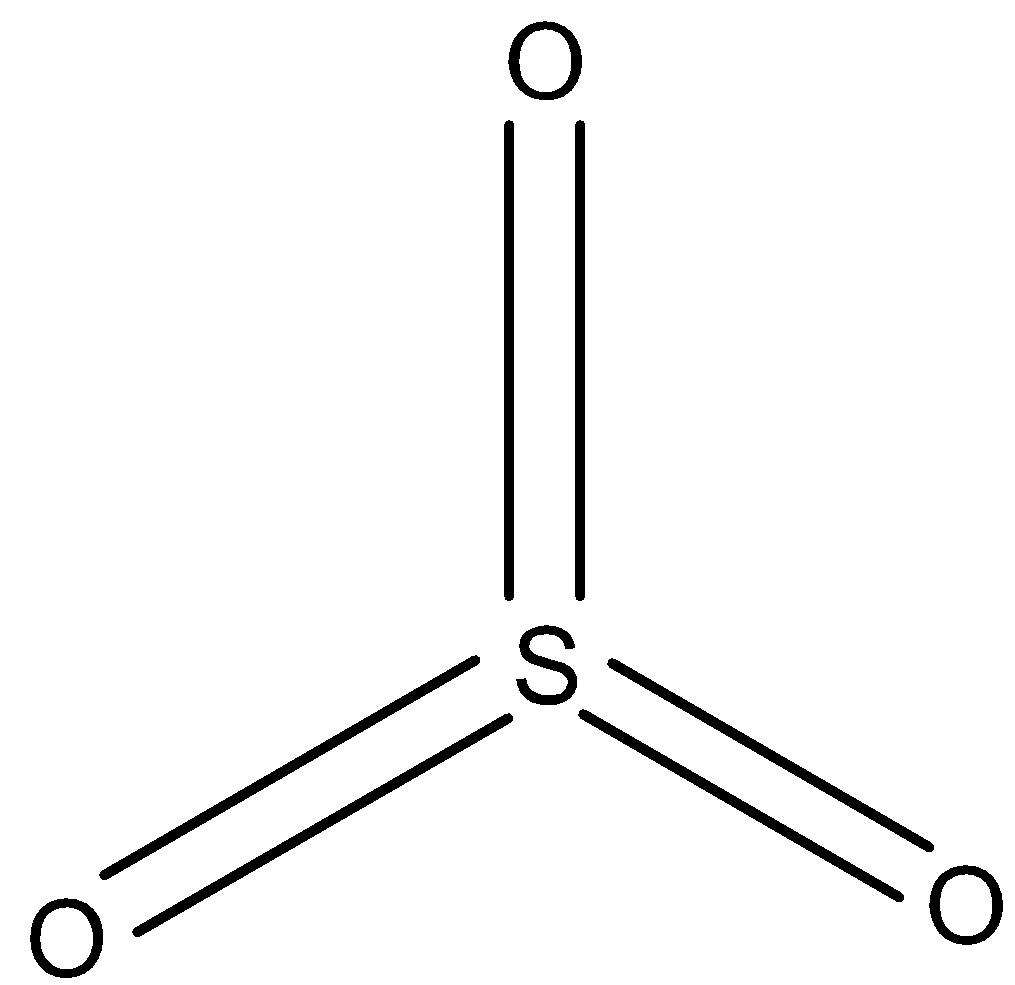
In $S{{O}_{3}}$, all the sulfur atoms are joined to oxygen atoms through a double bond as shown below. So the hybridization of sulfur in $S{{O}_{3}}$ is $s{{p}^{2}}$.
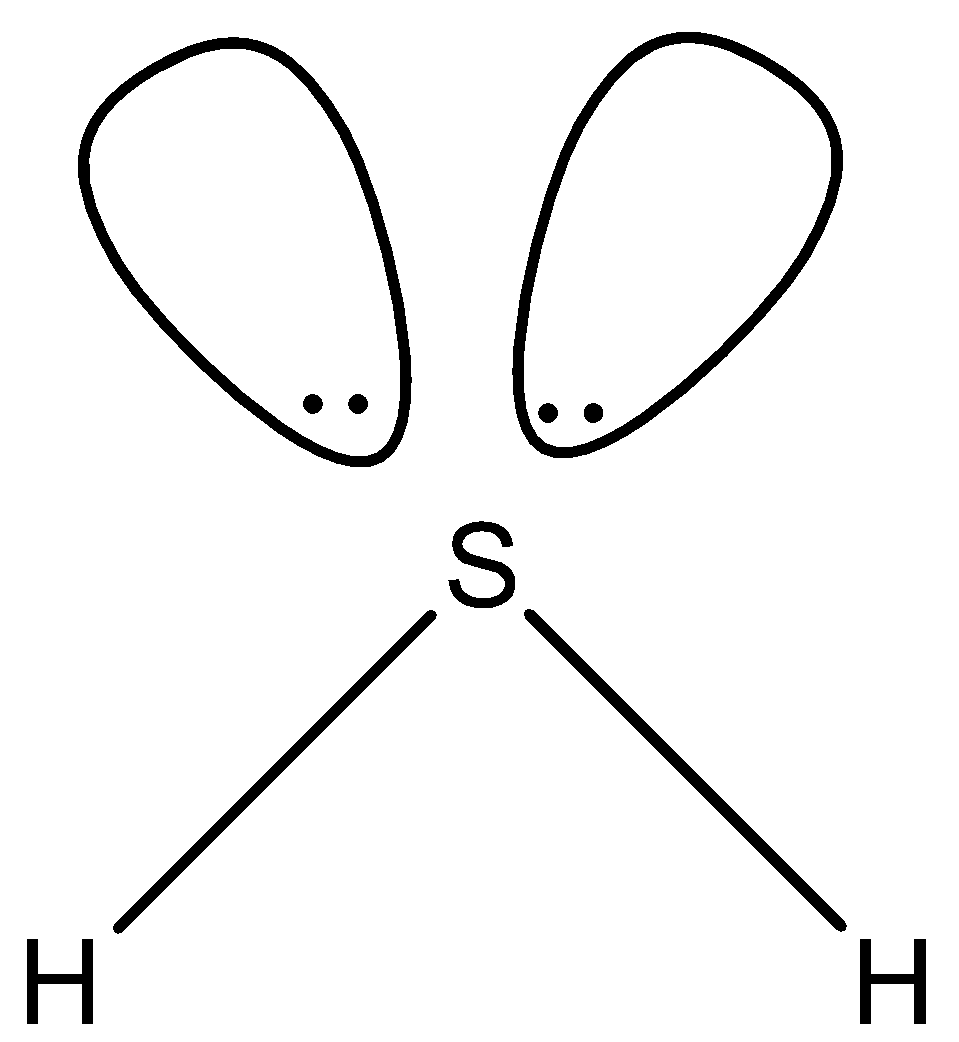
In ${{H}_{2}}S$, there are two single bonds and there are two lone pairs as shown below. So the hybridization of sulfur in ${{H}_{2}}S$ is $s{{p}^{3}}$.
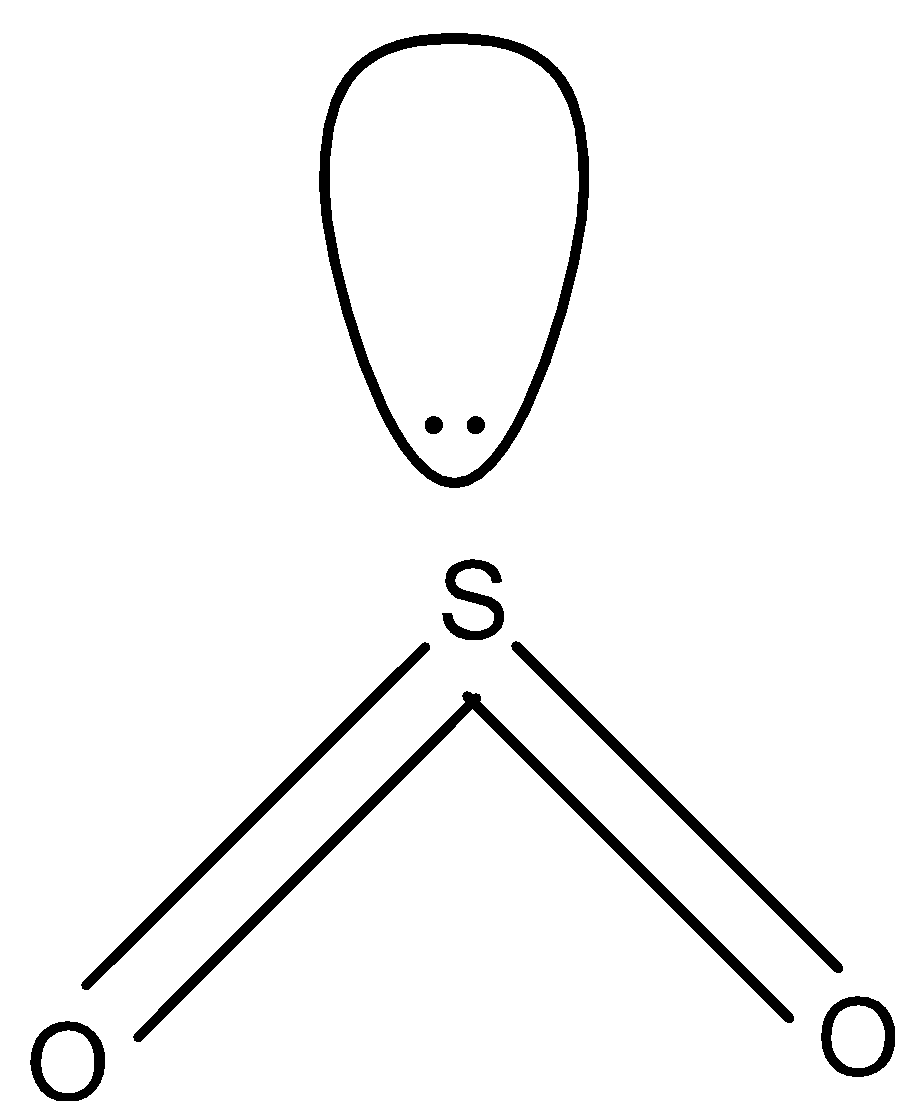
In $S{{O}_{2}}$, there are double bonds and there is one lone pair as shown below. So, the hybridization of sulfur in $S{{O}_{2}}$ is $s{{p}^{2}}$.
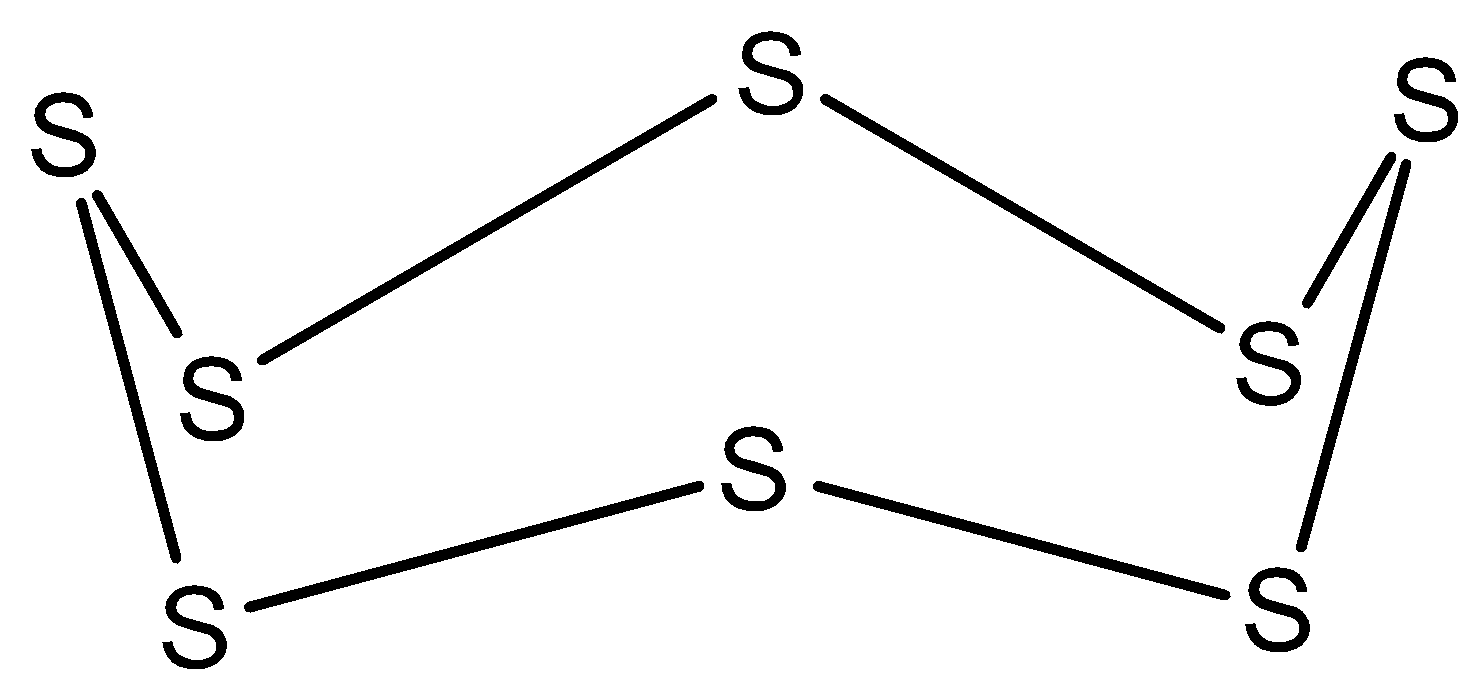
In ${{S}_{8}}$, all the bonds in this molecule are single as shown below. So, the hybridization of sulfur in ${{S}_{8}}$ is $s{{p}^{3}}$.
So, in compound (II) and (IV) the hybrid state is $s{{p}^{3}}$.
So, the correct answer is “Option C”.
Note: If the compound is having a double bond then the hybrid state will be $s{{p}^{2}}$ and if the compound is having a triple bond then the hybrid state will be $sp$.
Complete step by step solution:
In the compound, if the central atom should have $s{{p}^{3}}$-hybrid state, then all the bonds in the compound must be a single bond. If the compound has a double or triple bond then the central atom cannot have $s{{p}^{3}}$-hybrid state. If a single bond is not present, then the lone pair also contributes to $s{{p}^{3}}$-hybrid state.
In $S{{O}_{3}}$, all the sulfur atoms are joined to oxygen atoms through a double bond as shown below. So the hybridization of sulfur in $S{{O}_{3}}$ is $s{{p}^{2}}$.

In ${{H}_{2}}S$, there are two single bonds and there are two lone pairs as shown below. So the hybridization of sulfur in ${{H}_{2}}S$ is $s{{p}^{3}}$.

In $S{{O}_{2}}$, there are double bonds and there is one lone pair as shown below. So, the hybridization of sulfur in $S{{O}_{2}}$ is $s{{p}^{2}}$.

In ${{S}_{8}}$, all the bonds in this molecule are single as shown below. So, the hybridization of sulfur in ${{S}_{8}}$ is $s{{p}^{3}}$.

So, in compound (II) and (IV) the hybrid state is $s{{p}^{3}}$.
So, the correct answer is “Option C”.
Note: If the compound is having a double bond then the hybrid state will be $s{{p}^{2}}$ and if the compound is having a triple bond then the hybrid state will be $sp$.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




