
In which of the following reactions, the product is aromatic?
A.
B.
C.
D. All of these



Answer
514.5k+ views
Hint :Aromatic compound is identified from Huckel’s rule of aromaticity which states that for a compound to be aromatic, it must be a cyclic compound and all the carbon atoms present in the ring must be $ s{p^2} $ hybridized i.e., the ring must be planar and the ring should consist of a conjugation of $ (4n + 2)\pi $ electrons, where n is known as index of aromaticity and the value of $ n = 0,\,\;1,{\text{ 2, 3, }}... $ .
Complete Step By Step Answer:
Let us look at each given reaction separately.
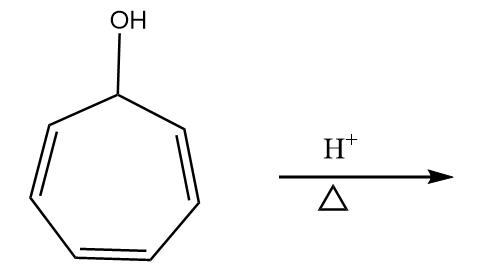
Reaction given in (A):
Step-1: The lone pair of electrons attract the hydrogen ion. The reaction proceeds as follows:
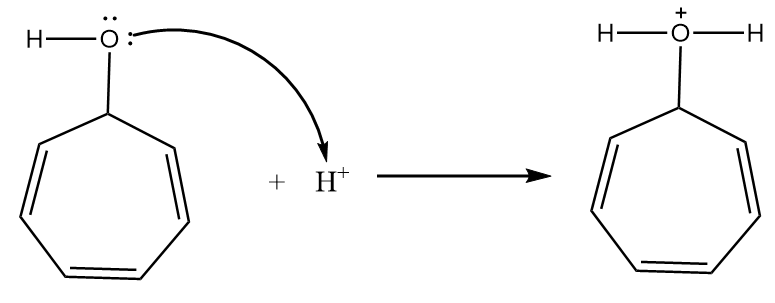
Step-2: When the compound is heated, then the removal of water molecule takes place and respective carbocation is formed. The reaction proceeds as follows:
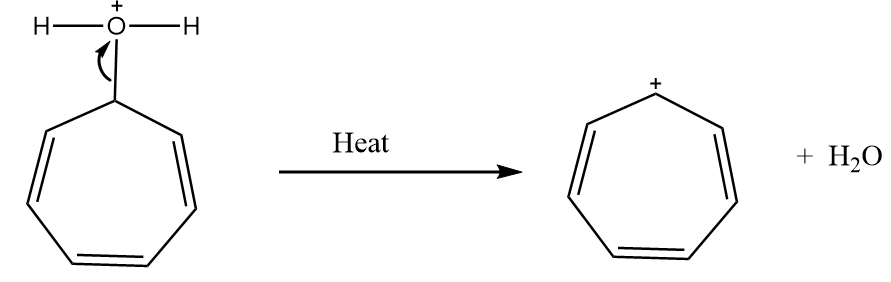
The compound formed is tropylium ion and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 6 $
$ \Rightarrow n = 1 $
So, the ion formed after the reaction is an aromatic compound.
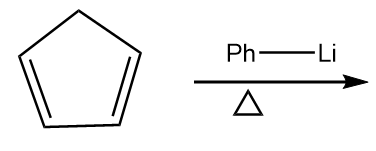
Reaction given in (B):
As the organolithium reagent which is used in the reaction is a very good base, so it extracts the acidic hydrogen in the presence of heat and respective carbanion is formed. The reaction proceeds as follows:
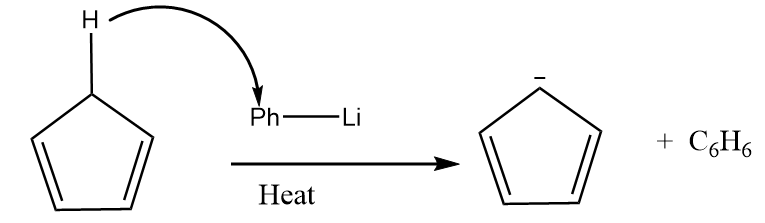
The compound formed is cyclopentadienyl anion and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 6 $
$ \Rightarrow n = 1 $
So, the ion formed after the reaction is an aromatic compound.
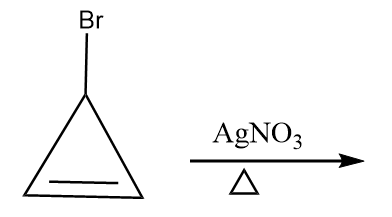
Reaction given in (C):
When a compound containing halide ion is heated in the presence of $ AgN{O_3} $ , then the formation of respective carbocation takes place along with the removal of silver halide as a precipitate. The given reaction proceeds as follows:
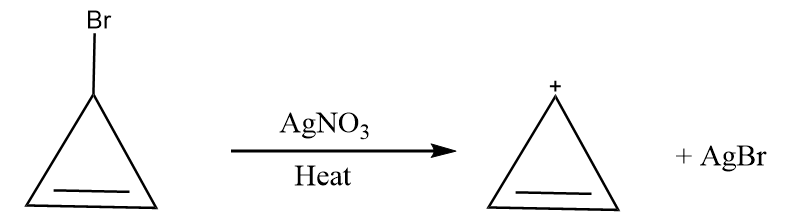
The compound formed is cyclopropyl cation and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 2 $
$ \Rightarrow n = 0 $
So, the ion formed after the reaction is an aromatic compound.
Thus, in all the given reactions an aromatic compound is formed as a product.
So, option (D) is the correct answer.
Note :
It is important to note that a compound with planar cyclic structure but consisting of a $ 4n\pi $ electron system is known as an antiaromatic compound whereas non-planar cyclic compounds are termed as non-aromatic compounds.
Complete Step By Step Answer:
Let us look at each given reaction separately.
Reaction given in (A):
Step-1: The lone pair of electrons attract the hydrogen ion. The reaction proceeds as follows:

Step-2: When the compound is heated, then the removal of water molecule takes place and respective carbocation is formed. The reaction proceeds as follows:

The compound formed is tropylium ion and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 6 $
$ \Rightarrow n = 1 $
So, the ion formed after the reaction is an aromatic compound.
Reaction given in (B):
As the organolithium reagent which is used in the reaction is a very good base, so it extracts the acidic hydrogen in the presence of heat and respective carbanion is formed. The reaction proceeds as follows:

The compound formed is cyclopentadienyl anion and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 6 $
$ \Rightarrow n = 1 $
So, the ion formed after the reaction is an aromatic compound.
Reaction given in (C):
When a compound containing halide ion is heated in the presence of $ AgN{O_3} $ , then the formation of respective carbocation takes place along with the removal of silver halide as a precipitate. The given reaction proceeds as follows:

The compound formed is cyclopropyl cation and it has following properties:
- It is a cyclic compound
- Ring is planar i.e.; all carbon atoms are $ s{p^2} $ hybridized
- The index of aromaticity for the compound i.e., $ 4n + 2 = 2 $
$ \Rightarrow n = 0 $
So, the ion formed after the reaction is an aromatic compound.
Thus, in all the given reactions an aromatic compound is formed as a product.
So, option (D) is the correct answer.
Note :
It is important to note that a compound with planar cyclic structure but consisting of a $ 4n\pi $ electron system is known as an antiaromatic compound whereas non-planar cyclic compounds are termed as non-aromatic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




