
In which of the following pairs indicated, the bond has less bond dissociation energy?
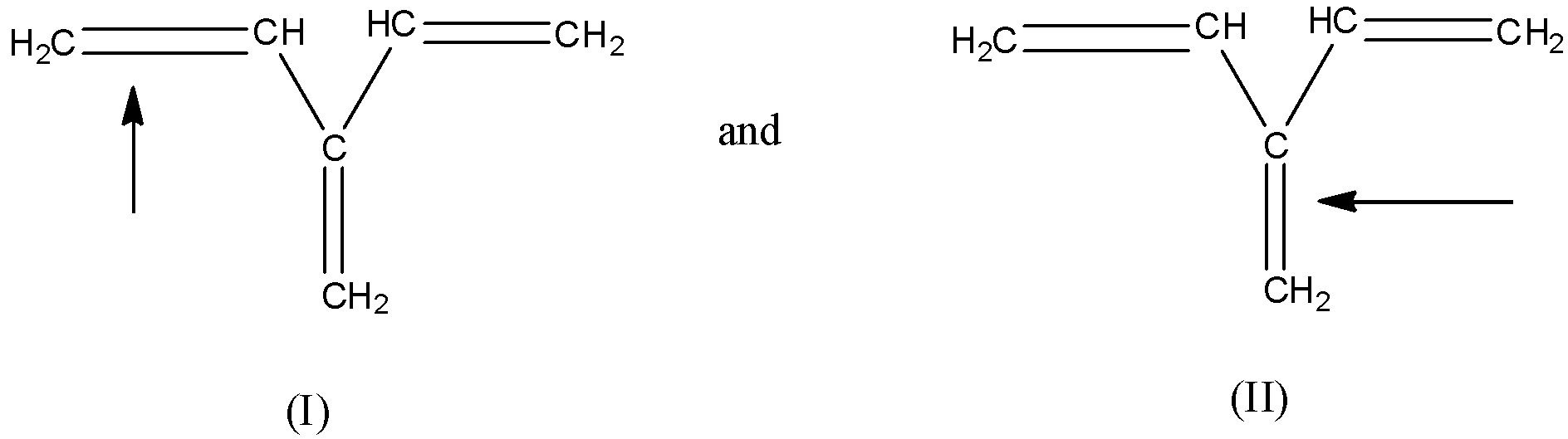
(a)- (I)
(b)- (II)
(c)- Both are equal
(d)- Cannot be determined
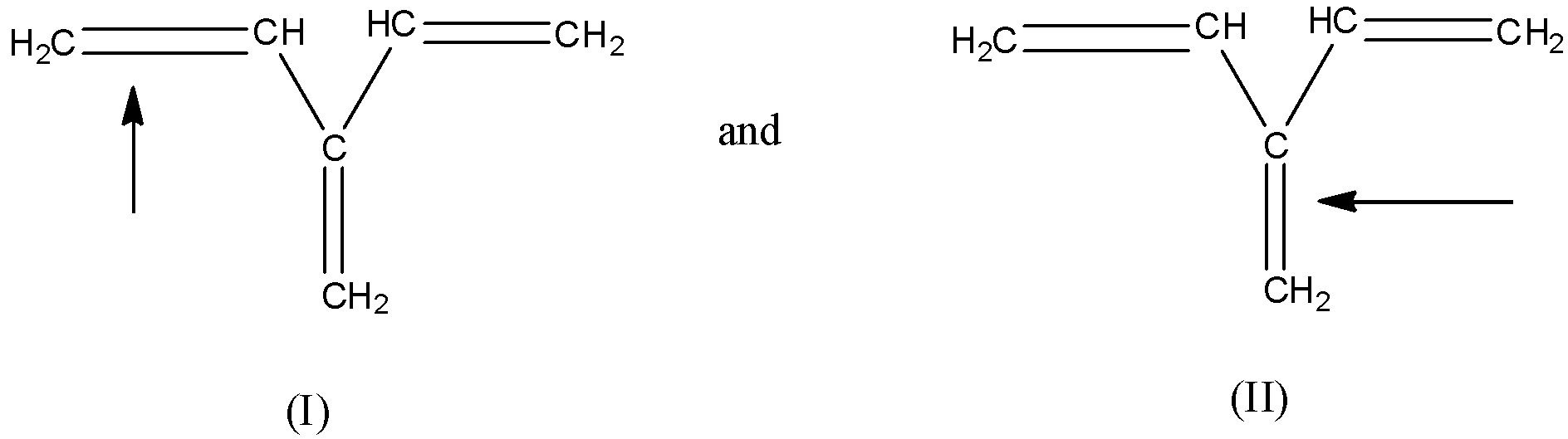
Answer
567.3k+ views
Hint: Bond dissociation energy is the energy required for the bond to break. The bond dissociation energy for the triple bond is greater than the double bond and the bond dissociation energy of the double bond is greater than the single bond.
Complete step by step solution:
Bond dissociation energy is the energy required for the bond to break. The stronger the bond, the more the energy will be required, so we can say that the bond dissociation energy for the triple bond is greater than the double bond and the bond dissociation energy of the double bond is greater than the single bond.
- In both the compounds, the double bonds have to be broken, so the bond dissociation energy has to be decided to the resonance factor.
- In compound (I), the double bond has only one single bond to show the conjugation, but in compound (II), the double has two single bonds to show the conjugation. So, in compound (I) the tendency of conjugation is lesser than the tendency of conjugation in compound (II) therefore, the double bond in compound (II) will be stronger than the double bond in compound (I).
- Hence, the bond dissociation energy required in the compound (I) will be lesser than the bond dissociation energy in compound (II).
So, the correct answer is “Option A”.
Note: As the resonance increases, the stability of the compound increases due to which more energy is required to break the compound. The bond dissociation energy of an ionic bond is more than the bond dissociation energy of the covalent bond.
Complete step by step solution:
Bond dissociation energy is the energy required for the bond to break. The stronger the bond, the more the energy will be required, so we can say that the bond dissociation energy for the triple bond is greater than the double bond and the bond dissociation energy of the double bond is greater than the single bond.
- In both the compounds, the double bonds have to be broken, so the bond dissociation energy has to be decided to the resonance factor.
- In compound (I), the double bond has only one single bond to show the conjugation, but in compound (II), the double has two single bonds to show the conjugation. So, in compound (I) the tendency of conjugation is lesser than the tendency of conjugation in compound (II) therefore, the double bond in compound (II) will be stronger than the double bond in compound (I).
- Hence, the bond dissociation energy required in the compound (I) will be lesser than the bond dissociation energy in compound (II).
So, the correct answer is “Option A”.
Note: As the resonance increases, the stability of the compound increases due to which more energy is required to break the compound. The bond dissociation energy of an ionic bond is more than the bond dissociation energy of the covalent bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




