
In which of the following pair hybridization of the central atoms are different?
A) \[Cl{{F}_{3}}O\]
B) \[Cl{{F}_{3}}O,\]
C) ${{\left[ Cl{{F}_{3}}O \right]}^{+}},{{\left[ Cl{{F}_{4}}O \right]}^{-}}$
D) ${{\left[ Cl{{F}_{4}}O \right]}^{-}},\left[ XeO{{F}_{4}} \right]$
Answer
574.2k+ views
Hint: Draw the structures of the each compounds
- Steric number rule is used to find the hybridization of a compound .
Complete Solution :
So here we have to find the pair of compounds which possess different hybridization. For finding the hybridization of a compound or complex we could use steric number rule and the formulae of steric number rule is,
-Steric number = Number of sigma bonds + Number of coordinate bonds +Number of lone pairs.
Steric number (S.N) = No. of $\sigma $bonds +No of coordinate bonds + No. of lone pairs (lp)
And for finding the steric number, we should know about the different types of bond and lone pair in a structure. So we have to draw the structure of each compound.
Steric number gives the hybridization and the geometry related to the hybridization, for example if the steric number is 2 then it is sp hybridized, if the S.N is 4 then it is $s{{p}^{3}}$ hybridized and so on
Now let’s draw the structure of each option and find the steric number and the hybridization related to the number.
Option (A) \[Cl{{F}_{3,}}Cl{{F}_{3}}O\]\[Cl{{F}_{3,}}Cl{{F}_{3}}O\]
Here for both the structure the central atom is chlorine , the valence of chlorine is 7
Structure of \[Cl{{F}_{3}}\]
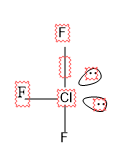
Here there are 3 $\sigma $+ 2 lp =5
S.N=5, hence the hybridization is $s{{p}^{3}}d$$s{{p}^{3}}d$
For \[Cl{{F}_{3}}O\]
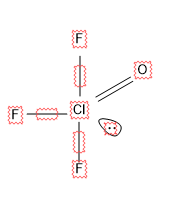
Here there are 4 sigma bonds and 1 lone pair.
So S.N = 4 + 1 = 5, hence hybridization is $s{{p}^{3}}d$
For option (A) both are having the same hybridization, so this is not the answer for the question.
Option (B) \[Cl{{F}_{3}}O,Cl{{F}_{3}}{{O}_{2}}\]
The structure and hybridization of \[Cl{{F}_{3}}O\] is mentioned above which is $s{{p}^{3}}d$ hybridized.
For \[Cl{{F}_{3}}{{O}_{2}}\]
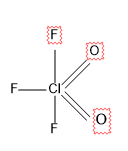
Here there are 5 sigma bonds and no lone pairs.
So the steric number is 5, which is $s{{p}^{3}}d$ hybridized.
Here also both the structures have the same hybridization and hence this also is not the answer.
Option (C) ${{\left[ Cl{{F}_{3}}O \right]}^{+}},{{\left[ Cl{{F}_{4}}O \right]}^{-}}$
Here also in both the structures Cl is the central atom
For ${{\left[ Cl{{F}_{3}}O \right]}^{+}}$
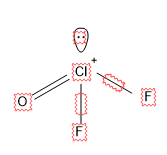
Here there is 1 lone pair and 3 sigma bonds.
So S.N = 1 + 3 = 4, so it is $s{{p}^{3}}$ hybridized.
For ${{\left[ Cl{{F}_{4}}O \right]}^{-}}$
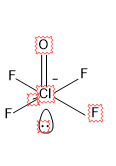
There are 5 sigma bonds and 1 lone pair, which is a total of 6.
So the steric number is 6, which means $s{{p}^{3}}{{d}^{2}}$ hybridization.
So both the structures have different hybridization, so this may be the answer, but we can confirm it by checking the final option too.
Option (D) ${{\left[ Cl{{F}_{4}}O \right]}^{-}},\left[ XeO{{F}_{4}} \right]$${{\left[ Cl{{F}_{4}}O \right]}^{-}},\left[ XeO{{F}_{4}} \right]$
The structure of ${{\left[ Cl{{F}_{4}}O \right]}^{-}}$ is given above and it has a steric number 6, with$s{{p}^{3}}{{d}^{2}}$ hybridization.
$\left[ XeO{{F}_{4}} \right]$ Has a structure like,
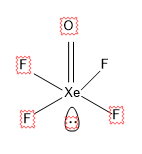 ,
,
Here there are 5 sigma bonds and 1 lone pair, which gives a total of 6, with $s{{p}^{3}}{{d}^{2}}$ hybridization.
So, the correct answer is “Option C”.
Note: Electronic configurations and the valency of each element should be known to draw the structures. Hybridization gives the geometry of each structure. And we should know that each atom forms which type of bonds and how many electrons they can share to form a bond.
- Steric number rule is used to find the hybridization of a compound .
Complete Solution :
So here we have to find the pair of compounds which possess different hybridization. For finding the hybridization of a compound or complex we could use steric number rule and the formulae of steric number rule is,
-Steric number = Number of sigma bonds + Number of coordinate bonds +Number of lone pairs.
Steric number (S.N) = No. of $\sigma $bonds +No of coordinate bonds + No. of lone pairs (lp)
And for finding the steric number, we should know about the different types of bond and lone pair in a structure. So we have to draw the structure of each compound.
Steric number gives the hybridization and the geometry related to the hybridization, for example if the steric number is 2 then it is sp hybridized, if the S.N is 4 then it is $s{{p}^{3}}$ hybridized and so on
Now let’s draw the structure of each option and find the steric number and the hybridization related to the number.
Option (A) \[Cl{{F}_{3,}}Cl{{F}_{3}}O\]\[Cl{{F}_{3,}}Cl{{F}_{3}}O\]
Here for both the structure the central atom is chlorine , the valence of chlorine is 7
Structure of \[Cl{{F}_{3}}\]

Here there are 3 $\sigma $+ 2 lp =5
S.N=5, hence the hybridization is $s{{p}^{3}}d$$s{{p}^{3}}d$
For \[Cl{{F}_{3}}O\]

Here there are 4 sigma bonds and 1 lone pair.
So S.N = 4 + 1 = 5, hence hybridization is $s{{p}^{3}}d$
For option (A) both are having the same hybridization, so this is not the answer for the question.
Option (B) \[Cl{{F}_{3}}O,Cl{{F}_{3}}{{O}_{2}}\]
The structure and hybridization of \[Cl{{F}_{3}}O\] is mentioned above which is $s{{p}^{3}}d$ hybridized.
For \[Cl{{F}_{3}}{{O}_{2}}\]

Here there are 5 sigma bonds and no lone pairs.
So the steric number is 5, which is $s{{p}^{3}}d$ hybridized.
Here also both the structures have the same hybridization and hence this also is not the answer.
Option (C) ${{\left[ Cl{{F}_{3}}O \right]}^{+}},{{\left[ Cl{{F}_{4}}O \right]}^{-}}$
Here also in both the structures Cl is the central atom
For ${{\left[ Cl{{F}_{3}}O \right]}^{+}}$

Here there is 1 lone pair and 3 sigma bonds.
So S.N = 1 + 3 = 4, so it is $s{{p}^{3}}$ hybridized.
For ${{\left[ Cl{{F}_{4}}O \right]}^{-}}$

There are 5 sigma bonds and 1 lone pair, which is a total of 6.
So the steric number is 6, which means $s{{p}^{3}}{{d}^{2}}$ hybridization.
So both the structures have different hybridization, so this may be the answer, but we can confirm it by checking the final option too.
Option (D) ${{\left[ Cl{{F}_{4}}O \right]}^{-}},\left[ XeO{{F}_{4}} \right]$${{\left[ Cl{{F}_{4}}O \right]}^{-}},\left[ XeO{{F}_{4}} \right]$
The structure of ${{\left[ Cl{{F}_{4}}O \right]}^{-}}$ is given above and it has a steric number 6, with$s{{p}^{3}}{{d}^{2}}$ hybridization.
$\left[ XeO{{F}_{4}} \right]$ Has a structure like,

Here there are 5 sigma bonds and 1 lone pair, which gives a total of 6, with $s{{p}^{3}}{{d}^{2}}$ hybridization.
So, the correct answer is “Option C”.
Note: Electronic configurations and the valency of each element should be known to draw the structures. Hybridization gives the geometry of each structure. And we should know that each atom forms which type of bonds and how many electrons they can share to form a bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




