
In which of the following molecules/species, all following characteristics are found?
(a) Tetrahedral hybridization.
(b) Hybridization can be considered to have taken place with the help of empty orbitals (s).
(c) All bond lengths are identical i.e., all A-B bond lengths are identical.
A) ${B_4}{H_6}$
B) $A{l_2}C{l_6}$
C) $BeC{l_3}\left( g \right)$
D) $B{F_4}^ - $
Answer
561.3k+ views
Hint: We have to remember that the Hybridization is that the concept in which atomic orbitals combine to make a new hybridized orbital which successively, influences molecular geometry and bonding properties.
We know that the electrons which are present at the outermost shell of an atom are called valence electrons and the valency of an electron is that the number of electrons through which atom accepts or donate to make a bond.
Complete step by step answer:
We can find the geometry of a molecule by finding the steric number of a molecule. The steric number of molecule can be calculated using the formula,
Steric number$ = \dfrac{{{\text{Valence electron of central atom}} + {\text{No}}{\text{.of bonded atom}} + {\text{Charge of compound}}}}{2}$
In \[B{F_4}^ - \] ion, all following characteristics are found.
Let us calculate the steric number of $B{F_4}^ - $ using the formula,
In case of $BF_4^ - $,
The valence electron of the central atom is 3.
Number surrounded atoms is 4.
The charge of the compound is -1.
We can substitute the values we get,
Steric number $ = \dfrac{{3\, + 4 + 1}}{2} = 4$
The steric number of $B{F_4}^ - $ is four. There are four bonded atoms and no lone pair around the central atom. Thus it is \[s{p^3}\] hybridized tetrahedral molecule. We can draw the structure of this compound as,
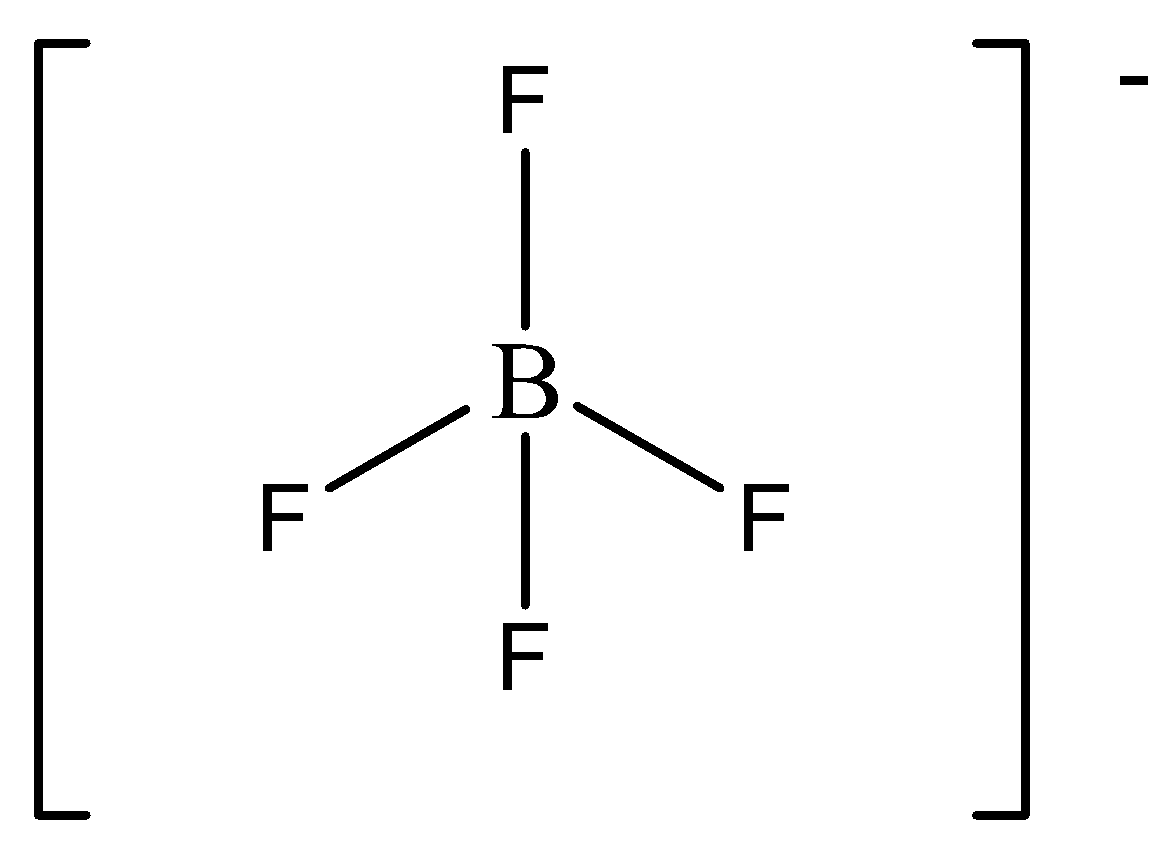
First, we discuss hybridization.
We have to remember that hybridization means the mixing of two atomic orbitals of the same energy to give a new hybrid molecular orbital of the same energy. Based on the type of orbitals involved, hybridization is classified as $sp$ , $s{p^2}$ , $s{p^3}$ , \[ds{p^2}\] , \[ds{p^3}\] etc. Hybridization Is often considered to have taken place with the assistance of an empty orbital. B contains 3 valence electrons, whereas 4 orbitals participate within the hybridization. Thus, one empty orbital participates within the hybridization.
(c) All the bond lengths are the same, i.e., all \[A - B\] bond lengths are identical as there are four identical \[B - F\] single bonds.
So, the correct answer is Option D.
Note: We can also calculate the steric number as follow,
The steric number is defined as the sum of the number of ligands and lone pairs surrounding the central atom.
${\text{Steric number}} = \left( {m + n} \right)$
The number of ligands is denoted as m and n is the number of lone pairs.
Example: Now we can calculate the steric number of ClF2- as,
${\text{Steric number}} = \left( {2 + 3} \right) = 5$
The steric number of ${\text{Cl}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is five ,which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized, two of them are in bonding and there is lone pairs thus it adopts the linear structure with the bond angle of ${\text{18}}{{\text{0}}^{\text{o}}}$.
We know that the electrons which are present at the outermost shell of an atom are called valence electrons and the valency of an electron is that the number of electrons through which atom accepts or donate to make a bond.
Complete step by step answer:
We can find the geometry of a molecule by finding the steric number of a molecule. The steric number of molecule can be calculated using the formula,
Steric number$ = \dfrac{{{\text{Valence electron of central atom}} + {\text{No}}{\text{.of bonded atom}} + {\text{Charge of compound}}}}{2}$
In \[B{F_4}^ - \] ion, all following characteristics are found.
Let us calculate the steric number of $B{F_4}^ - $ using the formula,
In case of $BF_4^ - $,
The valence electron of the central atom is 3.
Number surrounded atoms is 4.
The charge of the compound is -1.
We can substitute the values we get,
Steric number $ = \dfrac{{3\, + 4 + 1}}{2} = 4$
The steric number of $B{F_4}^ - $ is four. There are four bonded atoms and no lone pair around the central atom. Thus it is \[s{p^3}\] hybridized tetrahedral molecule. We can draw the structure of this compound as,

First, we discuss hybridization.
We have to remember that hybridization means the mixing of two atomic orbitals of the same energy to give a new hybrid molecular orbital of the same energy. Based on the type of orbitals involved, hybridization is classified as $sp$ , $s{p^2}$ , $s{p^3}$ , \[ds{p^2}\] , \[ds{p^3}\] etc. Hybridization Is often considered to have taken place with the assistance of an empty orbital. B contains 3 valence electrons, whereas 4 orbitals participate within the hybridization. Thus, one empty orbital participates within the hybridization.
(c) All the bond lengths are the same, i.e., all \[A - B\] bond lengths are identical as there are four identical \[B - F\] single bonds.
So, the correct answer is Option D.
Note: We can also calculate the steric number as follow,
The steric number is defined as the sum of the number of ligands and lone pairs surrounding the central atom.
${\text{Steric number}} = \left( {m + n} \right)$
The number of ligands is denoted as m and n is the number of lone pairs.
Example: Now we can calculate the steric number of ClF2- as,
${\text{Steric number}} = \left( {2 + 3} \right) = 5$
The steric number of ${\text{Cl}}{{\text{F}}_{\text{2}}}^{\text{ - }}$ is five ,which means that it is ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$ hybridized, two of them are in bonding and there is lone pairs thus it adopts the linear structure with the bond angle of ${\text{18}}{{\text{0}}^{\text{o}}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




