
In which of the following molecules, the number of possible \[\angle XAS\] angles are maximum in the anionic part of their solid state? [A: Central atom, X: surrounding atom]
A. $PB{r_5}$
B. ${N_2}{O_5}$
C. $PC{l_5}$
D. $C{l_2}{O_6}$
Answer
579k+ views
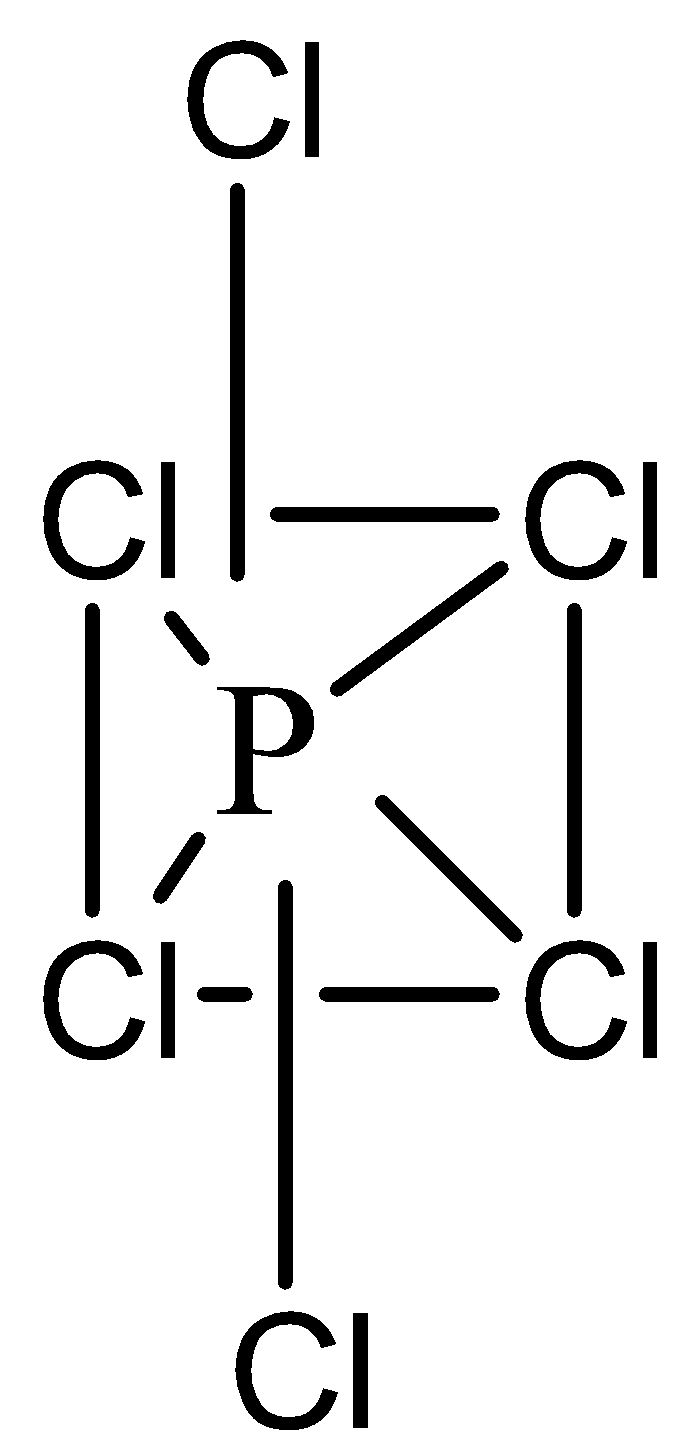
Hint: $PC{l_5}$ molecule has trigonal bipyramidal geometry which is unstable as axial \[P - Cl\] bonds are slightly larger than equatorial \[P - Cl\] bonds. Also, some bond angles are of \[{90^\circ }\] and others are of \[{120^\circ }\]. Due to this, trigonal bipyramidal geometry is not regular and not stable. Hence, in solid state, $PC{l_5}$
molecule dissociates into tetrahedral ${[PC{l_4}]^ + }$ and octahedral ${[PC{l_6}]^ - }$
$2PC{l_5}(s) \to {[PC{l_4}]^ + } + {[PC{l_6}]^ - }$
Complete step by step answer:
Actually $PB{r_5}$ is known to exist in solid state as a highly reactive yellow solid. Solid $PB{r_5}$ exists in the form of ${[PB{r_4}]^ + } + {[Br]^ - }$
$PB{r_5}(s) \to {[PB{r_4}]^ + } + {[Br]^ - }$
Both $PC{l_5}$ and $PB{r_5}$ trigonal bipyramidal geometry. This is not a regular structure and is not very stable. Therefore, $PC{l_5}$ will split up into more stable octahedral and tetrahedral structures which are stable than trigonal bipyramidal.
$2PC{l_5}(s) \to {[PC{l_4}]^ + } + {[PC{l_6}]^ - }(s{p^3}{d^2})$
On the other hand, $PB{r_5}$ splits up into stable tetrahedral structure as ${[PB{r_4}]^ + } + {[Br]^ - }$
$PB{r_5}(s) \to {[PB{r_4}]^ + } + {[Br]^ - }$
This splitting is different from $PC{l_5}$ because Br atoms are large and six atoms of Br cannot be easily accommodated around smaller P atoms.
$PC{l_5}$ has 2 angles of \[{90^\circ }\] and 3 angles of \[{120^\circ }\] (on the horizontal plane)
${N_2}{O_5}(s) \to N{O_2}^ + + N{O_3}^ - (s{p^2})$
$C{l_2}{O_6}(s) \to Cl{O_2}^ + + Cl{O_4}^ - (s{p^3})$
\[\angle XAX\] angles are maximum in the anionic part of their solid-state is $PC{l_5}$
Therefore, the correct answer is option (C).
Note: In solid state $PC{l_5}$ tries to exist as oppositely charged ions like ${[PC{l_4}]^ + }$ and ${[PC{l_6}]^ - }$ as the ionic bonding enhances the crystalline nature and also ${[PC{l_4}]^ + }$ is tetrahedral , while \[PC{l_6}^ - \]. is octahedral. These structures fit well into each other which gives more stability to solid structures.
molecule dissociates into tetrahedral ${[PC{l_4}]^ + }$ and octahedral ${[PC{l_6}]^ - }$
$2PC{l_5}(s) \to {[PC{l_4}]^ + } + {[PC{l_6}]^ - }$
Complete step by step answer:
Actually $PB{r_5}$ is known to exist in solid state as a highly reactive yellow solid. Solid $PB{r_5}$ exists in the form of ${[PB{r_4}]^ + } + {[Br]^ - }$
$PB{r_5}(s) \to {[PB{r_4}]^ + } + {[Br]^ - }$
Both $PC{l_5}$ and $PB{r_5}$ trigonal bipyramidal geometry. This is not a regular structure and is not very stable. Therefore, $PC{l_5}$ will split up into more stable octahedral and tetrahedral structures which are stable than trigonal bipyramidal.
$2PC{l_5}(s) \to {[PC{l_4}]^ + } + {[PC{l_6}]^ - }(s{p^3}{d^2})$
On the other hand, $PB{r_5}$ splits up into stable tetrahedral structure as ${[PB{r_4}]^ + } + {[Br]^ - }$
$PB{r_5}(s) \to {[PB{r_4}]^ + } + {[Br]^ - }$
This splitting is different from $PC{l_5}$ because Br atoms are large and six atoms of Br cannot be easily accommodated around smaller P atoms.
$PC{l_5}$ has 2 angles of \[{90^\circ }\] and 3 angles of \[{120^\circ }\] (on the horizontal plane)

${N_2}{O_5}(s) \to N{O_2}^ + + N{O_3}^ - (s{p^2})$
$C{l_2}{O_6}(s) \to Cl{O_2}^ + + Cl{O_4}^ - (s{p^3})$
\[\angle XAX\] angles are maximum in the anionic part of their solid-state is $PC{l_5}$
Therefore, the correct answer is option (C).
Note: In solid state $PC{l_5}$ tries to exist as oppositely charged ions like ${[PC{l_4}]^ + }$ and ${[PC{l_6}]^ - }$ as the ionic bonding enhances the crystalline nature and also ${[PC{l_4}]^ + }$ is tetrahedral , while \[PC{l_6}^ - \]. is octahedral. These structures fit well into each other which gives more stability to solid structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




