
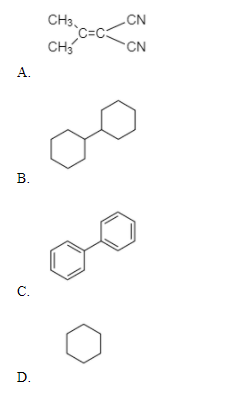
In which of the following molecules, all atoms are coplanar?

Answer
597.6k+ views
Hint: Coplanar objects are the objects which lie on the same plane whereas non-coplanar objects are the objects which do not lie in the same plane.
Complete step-by-step answer:
>In this compound, C=C is in-plane and the cyanide group attached to it will also be in the same plane but the ${ CH }_{ 2 }$ the group is ${ sp }^{ 3 }$ hybridized and the hydrogens are present above and below the plane. Hence, this compound will be non-planer.
>In this compound, all the atoms are ${ sp }^{ 3 }$ hybridized, so they have tetrahedral geometry. We know that in tetrahedral geometry, all the atoms are not in one plane. So, this compound is non-planer.
>In Biphenyl, all the carbon atoms are ${ sp }^{ 2 }$ hybridized. It undergoes nuclear substitution reaction, as one of the phenyl rings acts as electron-acceptor while the other will act as an electron-releasing group. Since all the hydrogens present are in one plane, this compound is coplanar.
>In this compound, all the atoms are ${ sp }^{ 3 }$ hybridized, so they have tetrahedral geometry. We know that in tetrahedral geometry, all the atoms are not in one plane. So, this compound is non-planer.
Hence, the correct option is C.
Note: The possibility to make a mistake is that you may choose option B. But in that compound, the carbon atoms are ${ sp }^{ 3 }$ hybridized, not ${ sp }^{ 2 }$ so they don’t lie in the same plane.
Complete step-by-step answer:
>In this compound, C=C is in-plane and the cyanide group attached to it will also be in the same plane but the ${ CH }_{ 2 }$ the group is ${ sp }^{ 3 }$ hybridized and the hydrogens are present above and below the plane. Hence, this compound will be non-planer.
>In this compound, all the atoms are ${ sp }^{ 3 }$ hybridized, so they have tetrahedral geometry. We know that in tetrahedral geometry, all the atoms are not in one plane. So, this compound is non-planer.
>In Biphenyl, all the carbon atoms are ${ sp }^{ 2 }$ hybridized. It undergoes nuclear substitution reaction, as one of the phenyl rings acts as electron-acceptor while the other will act as an electron-releasing group. Since all the hydrogens present are in one plane, this compound is coplanar.
>In this compound, all the atoms are ${ sp }^{ 3 }$ hybridized, so they have tetrahedral geometry. We know that in tetrahedral geometry, all the atoms are not in one plane. So, this compound is non-planer.
Hence, the correct option is C.
Note: The possibility to make a mistake is that you may choose option B. But in that compound, the carbon atoms are ${ sp }^{ 3 }$ hybridized, not ${ sp }^{ 2 }$ so they don’t lie in the same plane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




