
In which of the following, do all the carbon atoms not have the same hybridisation?
(This question has multiple correct options)
A.
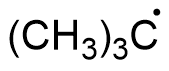
B.
C.
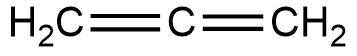
D.
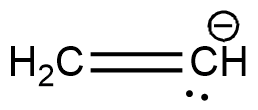
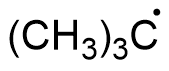

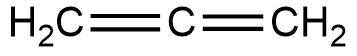
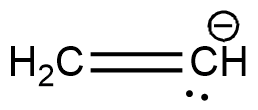
Answer
561k+ views
Hint: Hybridisation is the process of mixing the atomic orbitals of an atom to form a new set of identical orbitals called hybrid orbitals. The hybrid orbitals possess equivalent energy and shape.
Complete step by step answer:
In organic chemistry, a carbon attached via a single bond is $s{p^3}$ hybridised. Carbon attached via double and triple bonds are $s{p^2}$ and sp hybridised respectively. Also carbocation and carbon free radicals are $s{p^2}$ hybridised. A carbanion is $s{p^3}$ hybridised. Now let us discuss the hybridisation of each carbons in the given four options.
A.
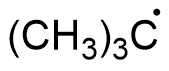
The structure of this compound can be drawn as,
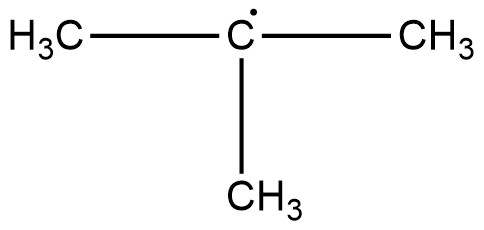
The three $C{H_3}$ carbons are $s{p^3}$ hybridised. These carbons are attached to four other atoms. The free radical carbon is $s{p^2}$ hybridised. It is surrounded by three other groups. Hence hybridisation of all the carbon atoms are not the same.
B.
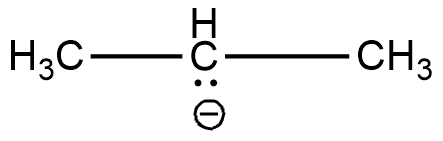
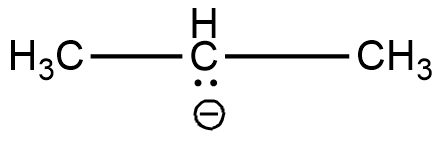
The two $C{H_3}$ carbons are $s{p^3}$ hybridised. The remaining is a carbon containing a negative charge. It is a carbanion. Carbanions are $s{p^3}$ hybridised. Hence all the carbons have the same hybridisation. Structure of this compound is shown below.
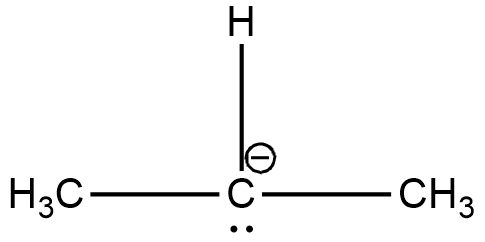
C.
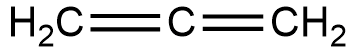
Structure of this compound is,
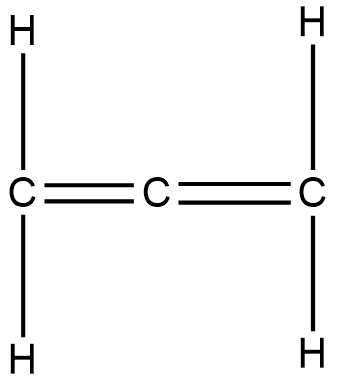
In this structure, the terminal carbons are attached via double bonds. Hence they are $s{p^2}$ hybridised. But the central atom is attached to two other atoms via two double bonds. Hence it is sp hybridised. Hence hybridisation of all carbons are not the same.
D.
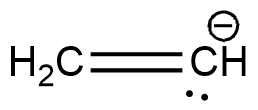
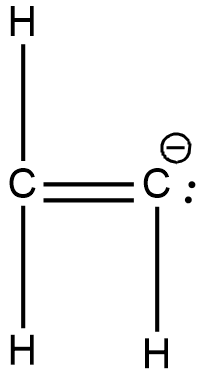
Both carbons are attached via a double bond. Hence they are $s{p^2}$ hybridised. All carbons have the same hybridisation.
In options A and C, all carbons do not have the same hybridisation. Hence correct options are A and C.
Note:
Hybridisation of carbon can be found using the number of atoms or groups to which it is attached. $s{p^3}$ carbon will be surrounded by four groups. Similarly, $s{p^2}$ and sp carbons are surrounded by three and two groups respectively. We can solve this question using this point also. Lone pair is also counted as an electron group.
Complete step by step answer:
In organic chemistry, a carbon attached via a single bond is $s{p^3}$ hybridised. Carbon attached via double and triple bonds are $s{p^2}$ and sp hybridised respectively. Also carbocation and carbon free radicals are $s{p^2}$ hybridised. A carbanion is $s{p^3}$ hybridised. Now let us discuss the hybridisation of each carbons in the given four options.
A.
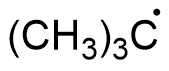
The structure of this compound can be drawn as,

The three $C{H_3}$ carbons are $s{p^3}$ hybridised. These carbons are attached to four other atoms. The free radical carbon is $s{p^2}$ hybridised. It is surrounded by three other groups. Hence hybridisation of all the carbon atoms are not the same.
B.

The two $C{H_3}$ carbons are $s{p^3}$ hybridised. The remaining is a carbon containing a negative charge. It is a carbanion. Carbanions are $s{p^3}$ hybridised. Hence all the carbons have the same hybridisation. Structure of this compound is shown below.

C.
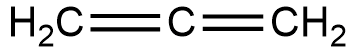
Structure of this compound is,

In this structure, the terminal carbons are attached via double bonds. Hence they are $s{p^2}$ hybridised. But the central atom is attached to two other atoms via two double bonds. Hence it is sp hybridised. Hence hybridisation of all carbons are not the same.
D.
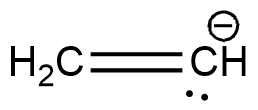

Both carbons are attached via a double bond. Hence they are $s{p^2}$ hybridised. All carbons have the same hybridisation.
In options A and C, all carbons do not have the same hybridisation. Hence correct options are A and C.
Note:
Hybridisation of carbon can be found using the number of atoms or groups to which it is attached. $s{p^3}$ carbon will be surrounded by four groups. Similarly, $s{p^2}$ and sp carbons are surrounded by three and two groups respectively. We can solve this question using this point also. Lone pair is also counted as an electron group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




