
In which of the following dative bonds is not present?
A. ${H_3}{O^ + }$
B. $N{H_4}^ + $
C. $A{l_2}C{l_6}$
D. ${N_2}{H_6}$
Answer
585k+ views
Hint: To get the correct answer of this problem first of all we need to know about dative bonds . Another name of dative bond is co- ordinate bond. It is a covalent bond (A kind of bond where a shared pair of electrons takes place) in which the electrons come from the same atom.
Complete step by step answer:
There are certain important points which are helpful in the identification of dative bonds. One point is that this bond is a lone pair in one atom and an empty orbital in the other atom. In dative covalent bonding the principle of sharing electrons between two atoms but in dative bonding the electrons involved in bonding are donated by the same atom. Let’s draw the structure of the given compounds and ions to check whether they have dative bonds or not. The structures of all four options are as follows.
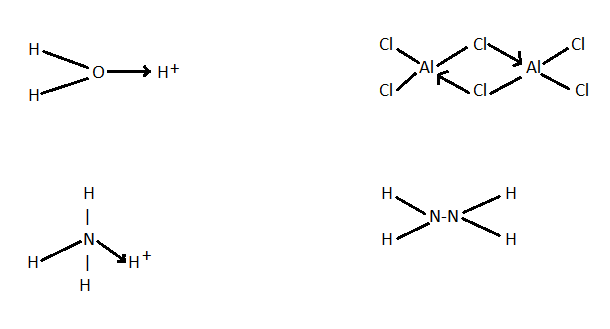
In the above structure we can see that among the hydronium ion /${H_3}{O^ + }$ , ammonium ion / $N{H_4}^ + $ , aluminium chloride / $A{l_2}C{l_6}$ and hydrazine / ${N_2}{H_6}$, only hydrazine do not have dative bond. So, the correct answer is “Option D”.
Note: Hence we need to draw the chemical structure of substance in order to get the dative bonding. Here in this question we have tried to complete the octet of atoms in the compounds by giving them correct electrons and then we get that only hydrazine does not have dative bonding. Other compounds have dative bonding in their structure and it is represented by arrow signs.
Complete step by step answer:
There are certain important points which are helpful in the identification of dative bonds. One point is that this bond is a lone pair in one atom and an empty orbital in the other atom. In dative covalent bonding the principle of sharing electrons between two atoms but in dative bonding the electrons involved in bonding are donated by the same atom. Let’s draw the structure of the given compounds and ions to check whether they have dative bonds or not. The structures of all four options are as follows.

In the above structure we can see that among the hydronium ion /${H_3}{O^ + }$ , ammonium ion / $N{H_4}^ + $ , aluminium chloride / $A{l_2}C{l_6}$ and hydrazine / ${N_2}{H_6}$, only hydrazine do not have dative bond. So, the correct answer is “Option D”.
Note: Hence we need to draw the chemical structure of substance in order to get the dative bonding. Here in this question we have tried to complete the octet of atoms in the compounds by giving them correct electrons and then we get that only hydrazine does not have dative bonding. Other compounds have dative bonding in their structure and it is represented by arrow signs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




