
In which of the following compounds is hydroxylic proton the most acidic:
A.
B.
C.
D.




Answer
570k+ views
Hint: In this question, we have to say which of the hydroxylic proton (the hydrogen of the \[ - OH\] or alcohol group) is mostly acidic. We can say it by the –I effect shown by the substituted halogen group in the chain
Complete step by step answer:
We will discuss the given options one by one in detail.
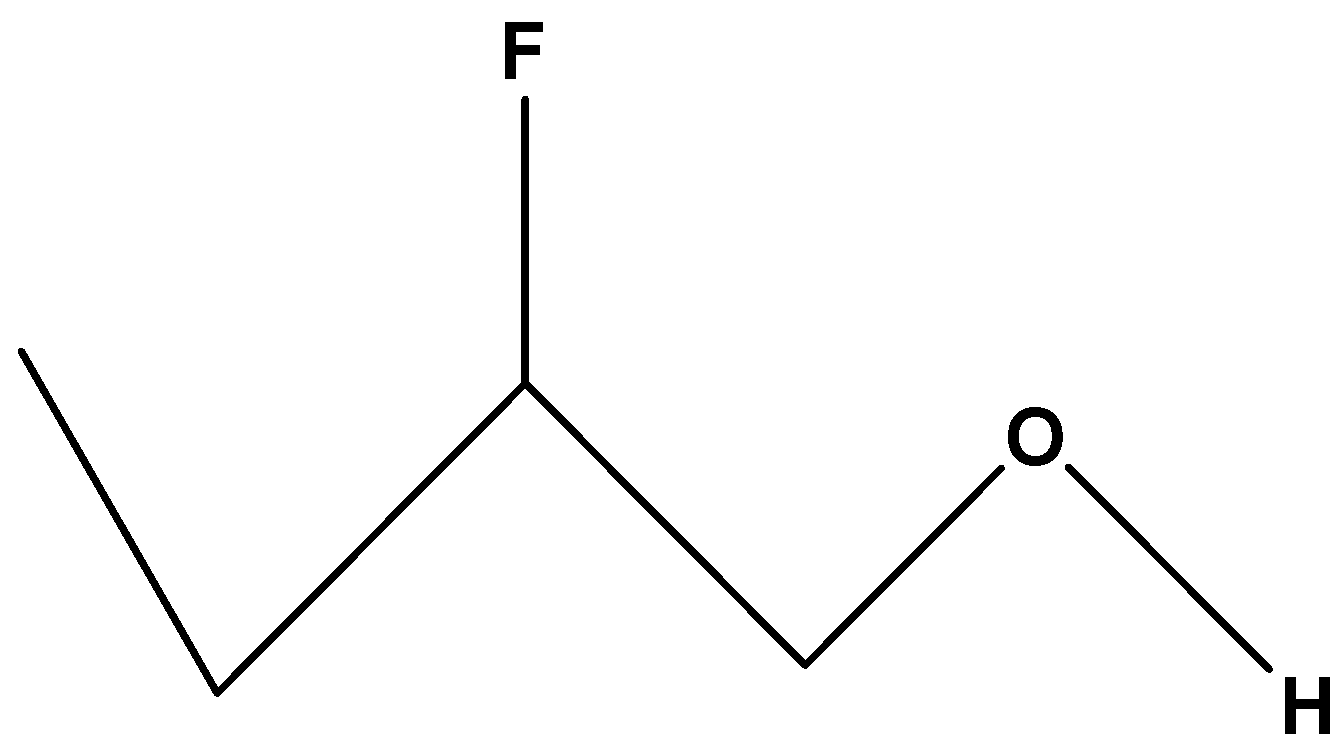
First we have the compound
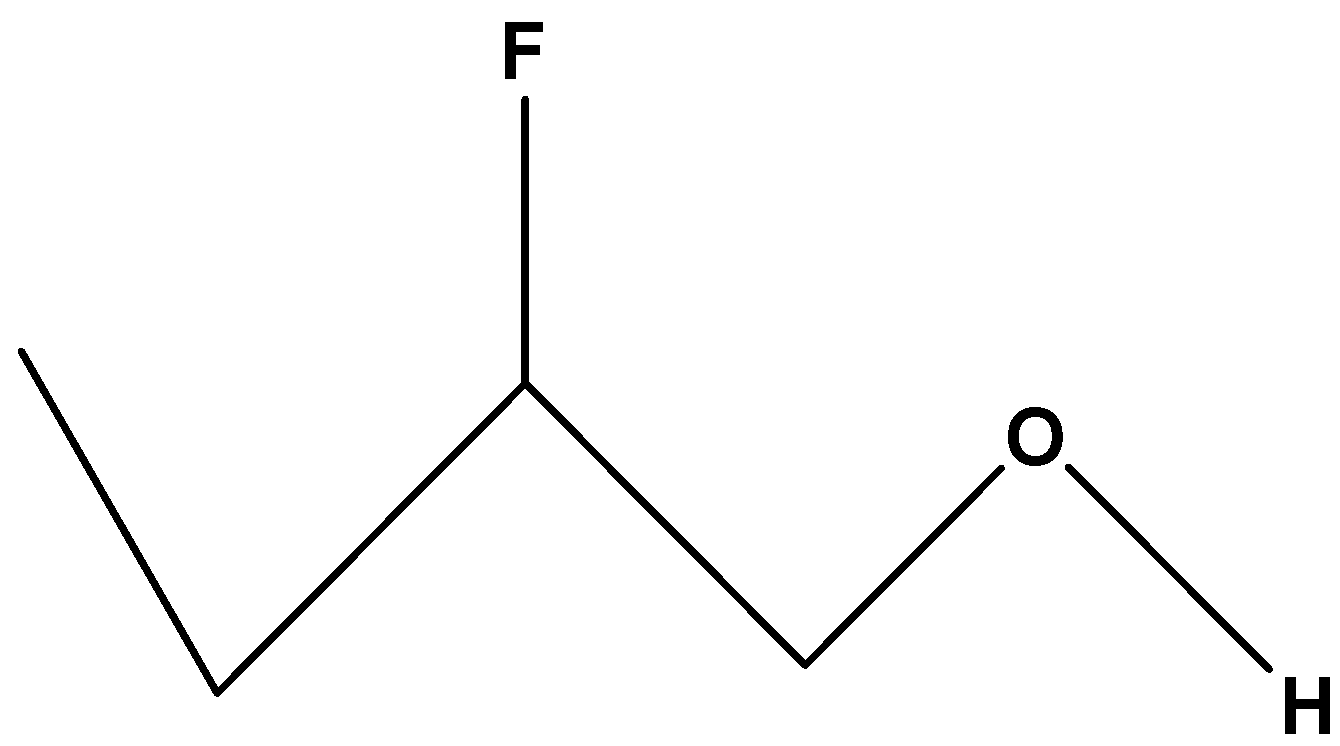
$C{H_3}C{H_2}CH\left( F \right)C{H_2}OH$. In these compounds, the substituted halogen group is fluorine which shows maximum –I effect among the other halogen. The effectiveness of fluorine increases with decrease in distance from the hydroxylic proton. Since the chain contains substituted Fluorine it will show more inductive effect or –I effect than the carbon chain containing iodine. The acidity of its hydroxylic proton will be intermediate.
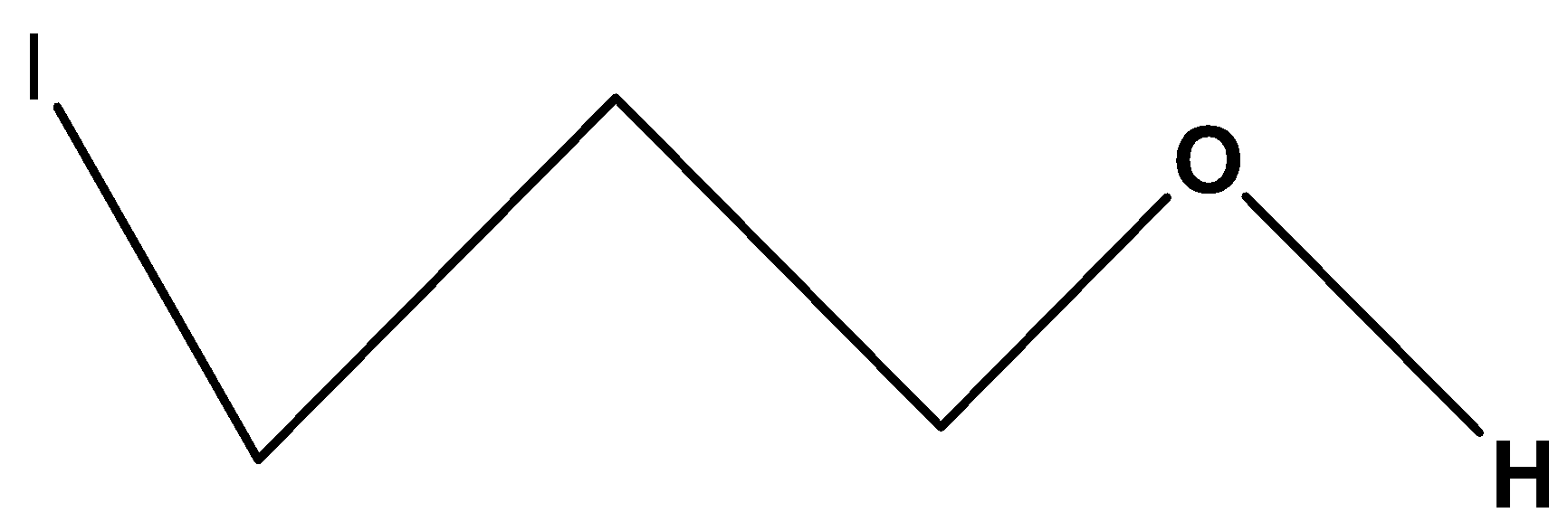
Second, we have the compound,
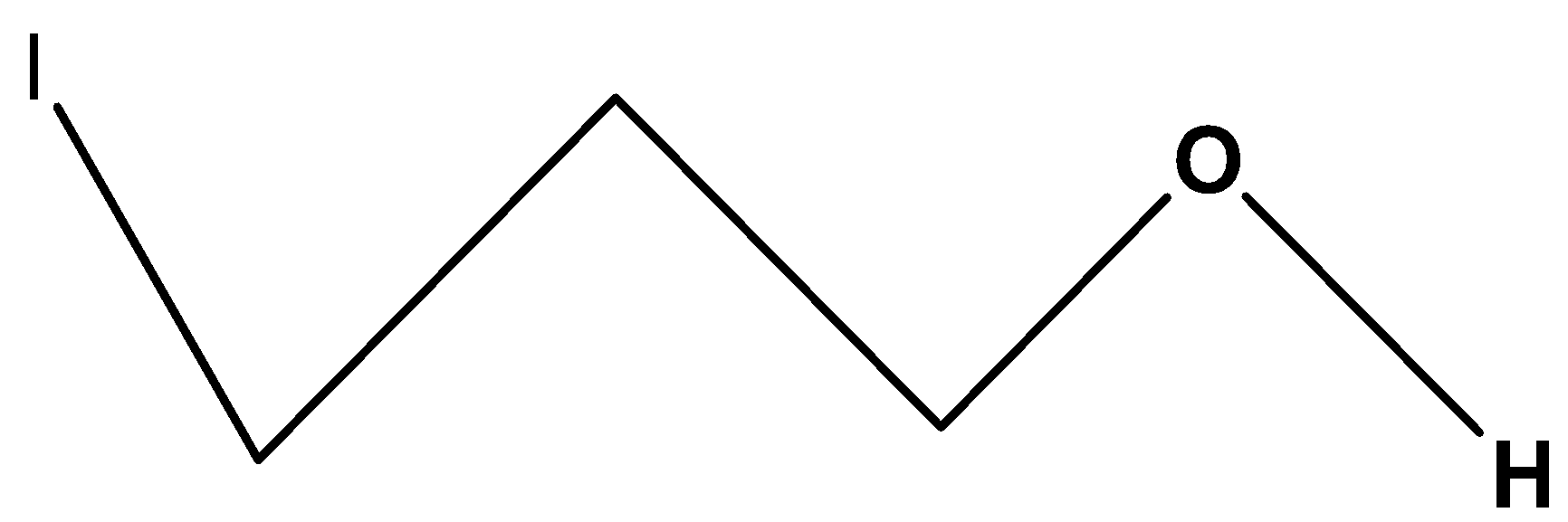
$C{H_2}\left( I \right)C{H_2}C{H_2}OH$. In these compounds, the substituted halogen group is iodine which shows less inductive effect or –I effect than the carbon chain containing fluorine group and also it is far from the hydroxylic proton. Its hydroxylic proton will be less acidic among the four compounds.

Third, we have the compound
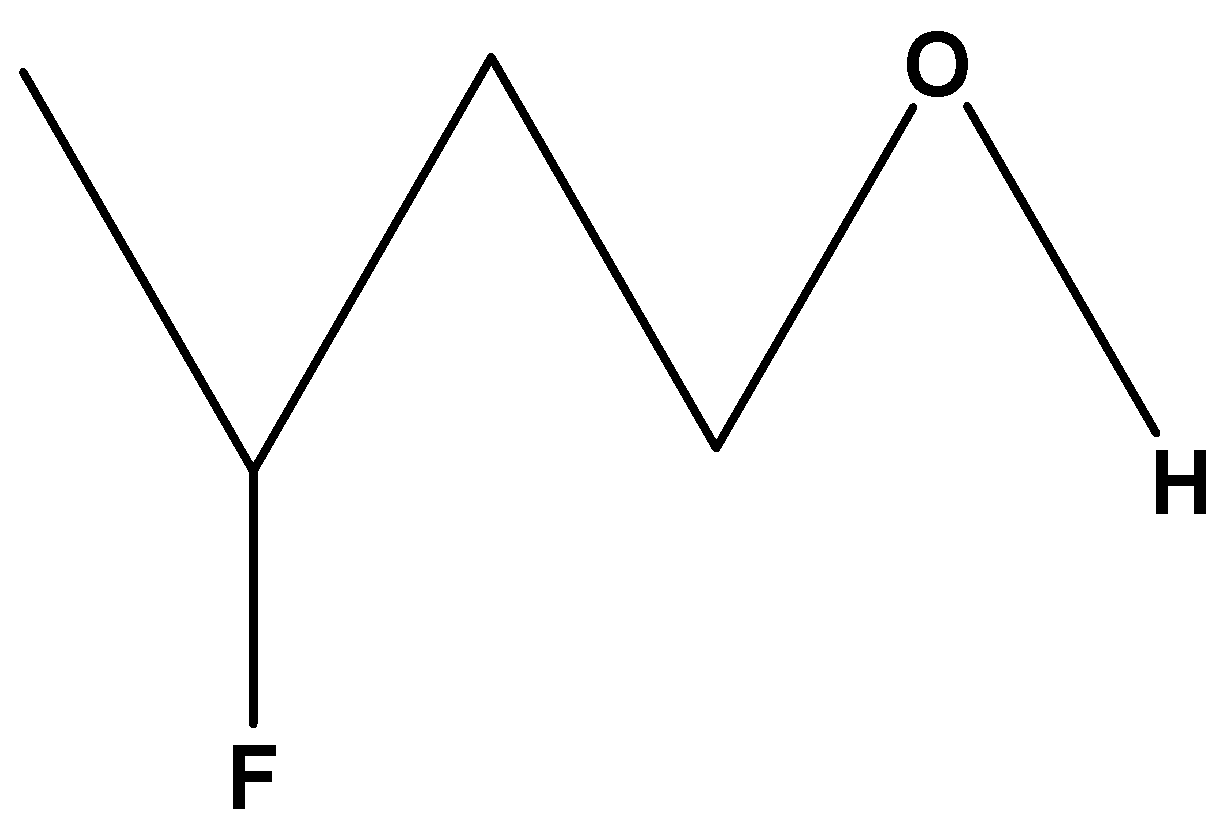
$C{H_3}CH\left( F \right)C{H_2}C{H_2}OH$ . In these compounds, fluorine is at the third carbon from the alcohol group and far away from the hydroxylic proton so its hydroxylic proton will be less acidic among the other two compounds containing fluorine.
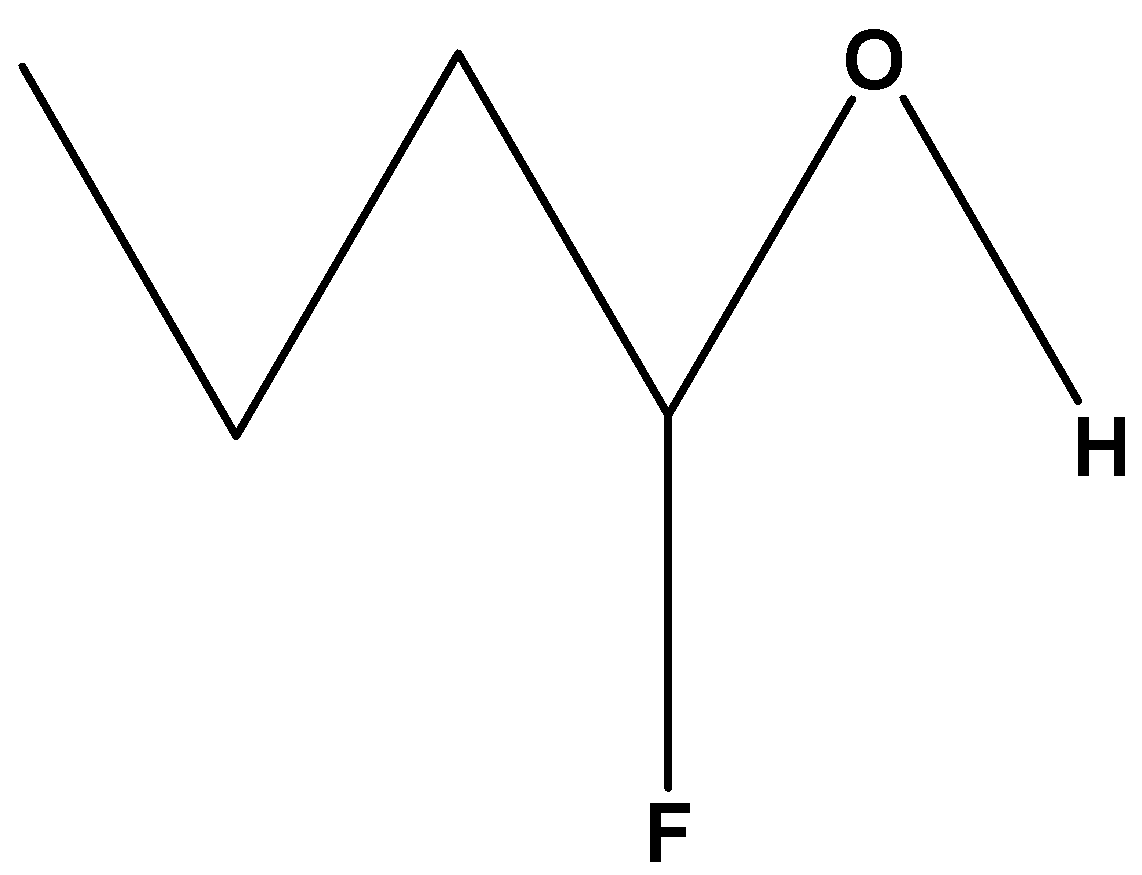
Fourth, we have the compound,
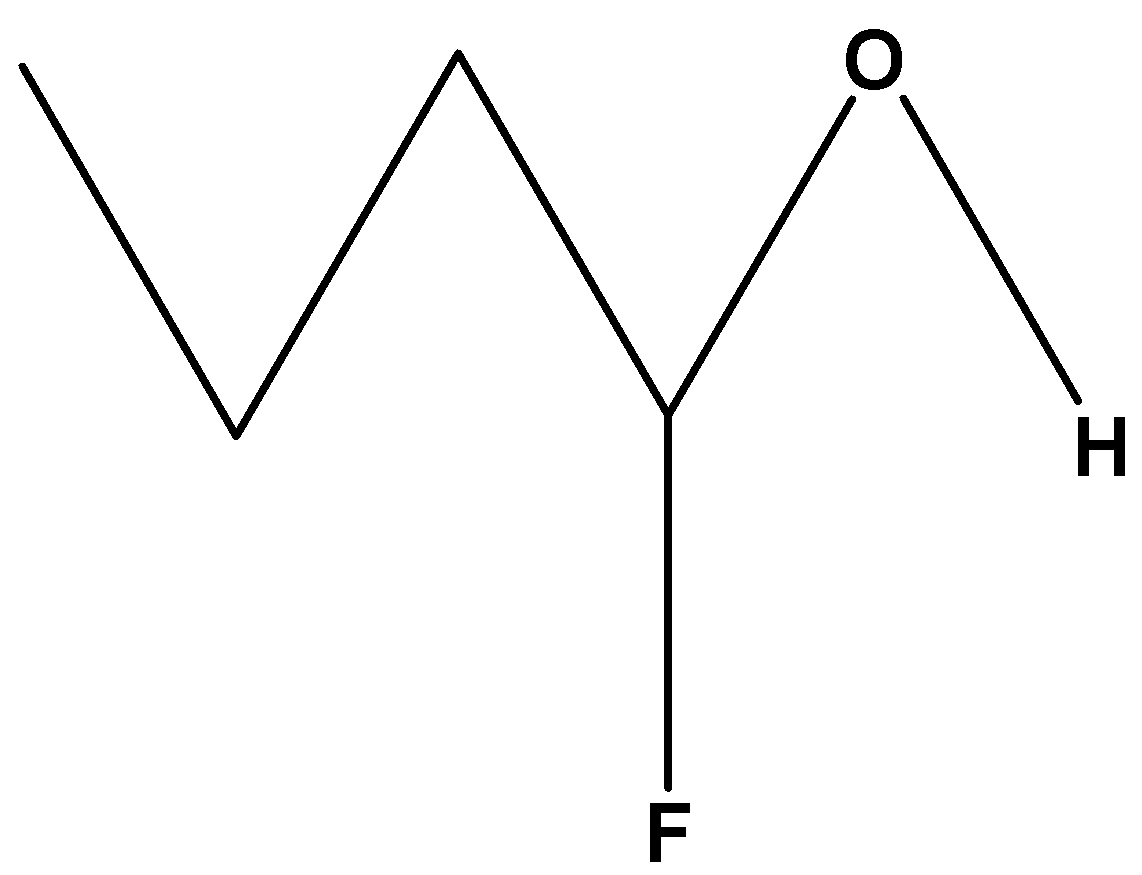
$C{H_3}C{H_2}C{H_2}CH\left( F \right)\left( {OH} \right)$. As these compounds contain fluorine which is attached at the first carbon chain and is nearer to the hydroxylic proton which will show maximum –I effect or inductive effect and has the most acidic hydroxylic proton.
So, the correct answer is Option D .
Note: −I effect is known as Inductive effect.Inductive effect refers to the arising of partial charges on molecule due to the unequal sharing of bonding electrons.Inductive effect is of two types: +I effect and –I effect.
Complete step by step answer:
We will discuss the given options one by one in detail.

First we have the compound
$C{H_3}C{H_2}CH\left( F \right)C{H_2}OH$. In these compounds, the substituted halogen group is fluorine which shows maximum –I effect among the other halogen. The effectiveness of fluorine increases with decrease in distance from the hydroxylic proton. Since the chain contains substituted Fluorine it will show more inductive effect or –I effect than the carbon chain containing iodine. The acidity of its hydroxylic proton will be intermediate.

Second, we have the compound,
$C{H_2}\left( I \right)C{H_2}C{H_2}OH$. In these compounds, the substituted halogen group is iodine which shows less inductive effect or –I effect than the carbon chain containing fluorine group and also it is far from the hydroxylic proton. Its hydroxylic proton will be less acidic among the four compounds.

Third, we have the compound
$C{H_3}CH\left( F \right)C{H_2}C{H_2}OH$ . In these compounds, fluorine is at the third carbon from the alcohol group and far away from the hydroxylic proton so its hydroxylic proton will be less acidic among the other two compounds containing fluorine.

Fourth, we have the compound,
$C{H_3}C{H_2}C{H_2}CH\left( F \right)\left( {OH} \right)$. As these compounds contain fluorine which is attached at the first carbon chain and is nearer to the hydroxylic proton which will show maximum –I effect or inductive effect and has the most acidic hydroxylic proton.
So, the correct answer is Option D .
Note: −I effect is known as Inductive effect.Inductive effect refers to the arising of partial charges on molecule due to the unequal sharing of bonding electrons.Inductive effect is of two types: +I effect and –I effect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




