
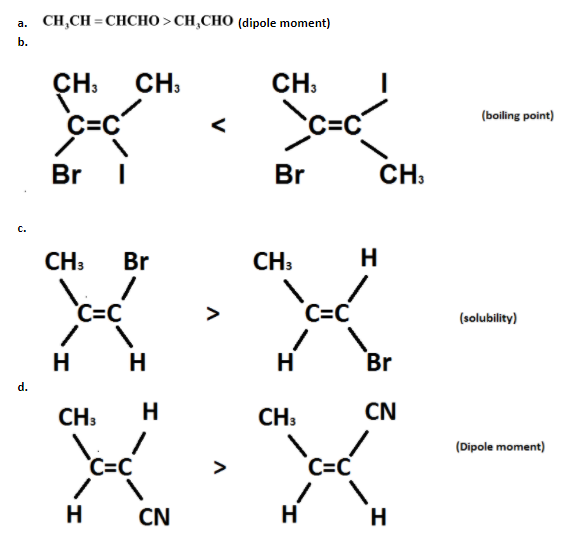
In which of the following cases /cases, is/are the order of indicated property correctly shown?
(this question has multiple correct options)

Answer
562.8k+ views
Hint: In the given question we will understand the effects of various properties such as dipole moment, boiling point, solubility in the case of the different reactions. So, we will know the concept of all these three effects with the help of given options.
Complete answer:
Dipole moments take place when separation of charge occurs between two ions in an ionic bond.
So, in case the dipole moment of crotonaldehyde is more than the dipole moment of acetaldehyde.
In this case Symmetricity occurs. As Symmetricity makes the boiling point lower so boiling point of the dihalo derivative of cis alkene is higher than dihalo derivative of trans alkene. therefore, trans has more symmetricity than cis.
Due to the symmetricity Trans alkenes have lower solubility so the solubility of the bromo derivative of cis alkene is lesser than the bromo derivative of trans alkene.
As the dipole value in trans increases so the dipole moment of the cyano derivative of trans alkene is more than the dipole moment of the cyano derivative of cis alkene.
Hence, the options a and d correctly represent the order of properties.
Note: As boiling point and solubility come under the physical properties. And dipole moment statesDipole moments tell us about the charge separation in a molecule. It is the measure of net molecular polarity, that is magnitude of the charge at end of the molecular dipole times distance between the charges.
Complete answer:
Dipole moments take place when separation of charge occurs between two ions in an ionic bond.
So, in case the dipole moment of crotonaldehyde is more than the dipole moment of acetaldehyde.
In this case Symmetricity occurs. As Symmetricity makes the boiling point lower so boiling point of the dihalo derivative of cis alkene is higher than dihalo derivative of trans alkene. therefore, trans has more symmetricity than cis.
Due to the symmetricity Trans alkenes have lower solubility so the solubility of the bromo derivative of cis alkene is lesser than the bromo derivative of trans alkene.
As the dipole value in trans increases so the dipole moment of the cyano derivative of trans alkene is more than the dipole moment of the cyano derivative of cis alkene.
Hence, the options a and d correctly represent the order of properties.
Note: As boiling point and solubility come under the physical properties. And dipole moment statesDipole moments tell us about the charge separation in a molecule. It is the measure of net molecular polarity, that is magnitude of the charge at end of the molecular dipole times distance between the charges.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




