
In which of the below given cells, the potential difference between the terminals of a cell exceeds its value of emf?
A.
B.
C.
D.




Answer
579.9k+ views
Hint: Terminal potential difference of a cell is described as the potential difference between two electrodes of a cell which is in a closed circuit. If the value of terminal potential difference of a cell is given as less than the emf of a cell, if the current is drawn from the cell which means during the discharging of the cell. These all may help you to solve this question.
Complete answer:
The terminal potential of each of the cells should be calculated. Terminal potential difference of a cell is described as the potential difference between the two electrodes of a cell in a closed circuit.
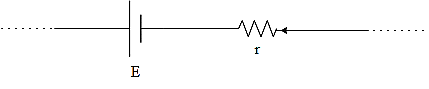
That is for the battery shown in (a), the terminal potential difference will be,
$V=E-ir$
Where $E$be the emf of the cell, \[i\] be the current through the cell and \[r\] be the internal resistance of the cell.
This is because the both ends are open and the current is coming first to the resistance.
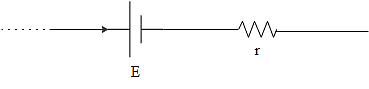
The terminal potential for the cell in (b) will be,
\[V=E+ir\]
Only one of the ends is open here and the current approaches the battery first.
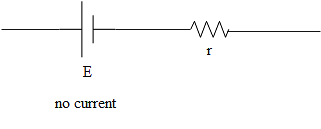
The terminal potential of the cell in (c) is,
\[V=E\]
As there is no current in the cell.
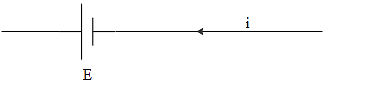
And the terminal potential of the cell in (d) will be,
\[V=E\]
As the internal resistance of the cell is zero.
So, the correct answer is “Option B”.
Note:
The value of potential difference of the terminals of a cell becomes greater than the emf of the cell during charging the cell. That is when the positive electrode of the cell is connected to the positive terminal of the battery charger and the negative electrode of the cell is connected to the negative terminal of the battery charger.
Complete answer:
The terminal potential of each of the cells should be calculated. Terminal potential difference of a cell is described as the potential difference between the two electrodes of a cell in a closed circuit.
That is for the battery shown in (a), the terminal potential difference will be,
$V=E-ir$
Where $E$be the emf of the cell, \[i\] be the current through the cell and \[r\] be the internal resistance of the cell.
This is because the both ends are open and the current is coming first to the resistance.
The terminal potential for the cell in (b) will be,
\[V=E+ir\]
Only one of the ends is open here and the current approaches the battery first.
The terminal potential of the cell in (c) is,
\[V=E\]
As there is no current in the cell.
And the terminal potential of the cell in (d) will be,
\[V=E\]
As the internal resistance of the cell is zero.
So, the correct answer is “Option B”.
Note:
The value of potential difference of the terminals of a cell becomes greater than the emf of the cell during charging the cell. That is when the positive electrode of the cell is connected to the positive terminal of the battery charger and the negative electrode of the cell is connected to the negative terminal of the battery charger.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




