
In what respect do three isotopes of hydrogen differ? Give them structure?
Answer
481.5k+ views
Hint: Atomic number 1 having the symbol H represents the chemical element hydrogen. The lightest Known element in the periodic table is hydrogen with standard atomic weight 1.008. Hydrogen exists as a diatomic molecule known as dihydrogen. Hydrogen was discovered by Henry Cavendish.
Complete Step By Step Answer:
There are 3 naturally occurring isotopes of hydrogen that are $ _1^1H{\text{ ,}}_1^2{\text{H, }}_1^3{\text{H}} $ .
$ ^3H $ is known as tritium.
$ ^2H $ is known as Deuterium and $ ^1H $ is known as Protium.
$ ^1H,{{\text{ }}^2}{\text{H}} $ are stable isotopes and $ ^3H $ has a 12.32 years of half-life.
One proton and no neutron are present in the protium.
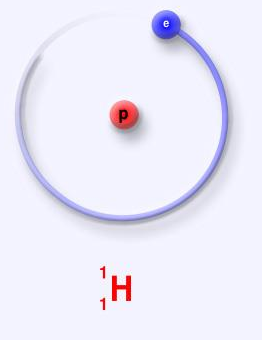
A single positively charged proton and a single negatively charged electron bound to the nucleus by Coulomb force are present in an electrically neutral atom. 1.007825 u is the relative atomic mass of protium, It is used for the treatment of intestine and stomach-related illnesses.
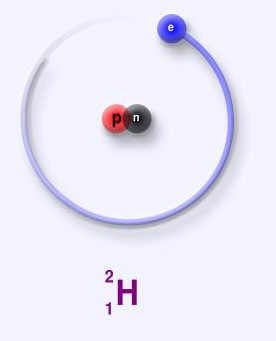
One proton and one neutron are present in the nucleus of Deuterium. The nucleus of deuterium is known as deuteron. Mass of deuterium is 2.014102 u. Heavy water is a type of water that is enriched with deuterium molecules. In commercial nuclear fusion, Deuterium is a potential fuel.
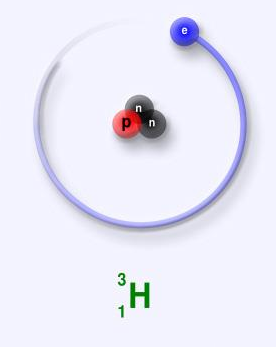
Tritium carries one proton, two neutrons in its nucleus. 3.01604928 u is the atomic mass of the tritium. Tritium is an extremely infrequent naturally occurring element on the earth. It can be produced artificially by irradiating either lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and hence it is a low abundance by-product in normal operations of the nuclear reactor.
Note:
Atoms of the same elements have differences in the number of neutrons but have the same number of protons and electrons are called isotopes. Due to the difference in the number of neutrons in the various isotopes of an element the isotopes have different masses. Moreover, isotopes have the same atomic number.
Complete Step By Step Answer:
There are 3 naturally occurring isotopes of hydrogen that are $ _1^1H{\text{ ,}}_1^2{\text{H, }}_1^3{\text{H}} $ .
$ ^3H $ is known as tritium.
$ ^2H $ is known as Deuterium and $ ^1H $ is known as Protium.
$ ^1H,{{\text{ }}^2}{\text{H}} $ are stable isotopes and $ ^3H $ has a 12.32 years of half-life.
One proton and no neutron are present in the protium.

A single positively charged proton and a single negatively charged electron bound to the nucleus by Coulomb force are present in an electrically neutral atom. 1.007825 u is the relative atomic mass of protium, It is used for the treatment of intestine and stomach-related illnesses.
One proton and one neutron are present in the nucleus of Deuterium. The nucleus of deuterium is known as deuteron. Mass of deuterium is 2.014102 u. Heavy water is a type of water that is enriched with deuterium molecules. In commercial nuclear fusion, Deuterium is a potential fuel.

Tritium carries one proton, two neutrons in its nucleus. 3.01604928 u is the atomic mass of the tritium. Tritium is an extremely infrequent naturally occurring element on the earth. It can be produced artificially by irradiating either lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and hence it is a low abundance by-product in normal operations of the nuclear reactor.

Note:
Atoms of the same elements have differences in the number of neutrons but have the same number of protons and electrons are called isotopes. Due to the difference in the number of neutrons in the various isotopes of an element the isotopes have different masses. Moreover, isotopes have the same atomic number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




