
In this sequence of reaction A, B, C and D are respectively:
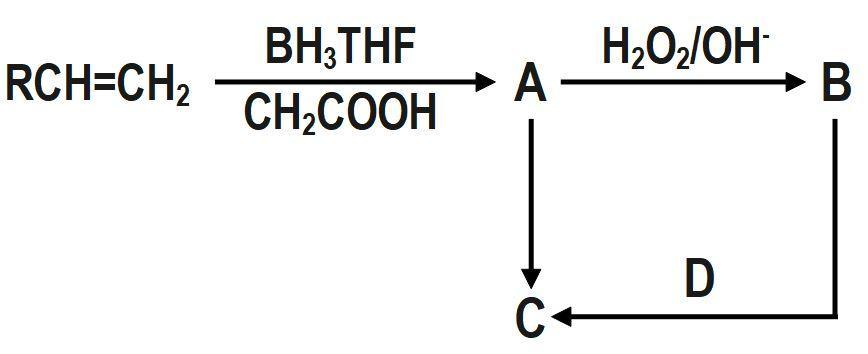
(A) $ \left[ {{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B \right],\left[ RC{{H}_{2}}C{{H}_{2}}OH \right],\left[ RC{{H}_{2}}C{{H}_{3}} \right],\left[ HI \right] $
(B) $ \left[ {{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B \right],\left[ RCH(OH)C{{H}_{3}} \right],\left[ RC{{H}_{2}}C{{H}_{3}} \right],\left[ HI \right] $
(C) $ \left[ {{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B \right],\left[ RCH(OH)C{{H}_{2}}C{{H}_{3}} \right],\left[ RC{{H}_{2}}C{{H}_{3}} \right],\left[ HI \right] $
(D) None of the above.

Answer
514.2k+ views
Hint :We know that alcohol is derivatives of hydrocarbons whose functional group is $ -OH $ as $ -OH $ has replaced a hydrogen atom. Depending on the presence of hydroxyl groups in the compound there are different types of alcohol i.e. primary alcohol, secondary alcohol, and tertiary alcohol.
Complete Step By Step Answer:
When hydroxyl group atoms form a bond with saturated carbon atoms, alcohol is formed. Now, if the dehydration of alcohol takes place, then we get ether. In this chapter, you will get to learn how these compounds are connected to one another. In addition to this, there are three types of alcohols: Monohydric, Dihydric, and Trihydric alcohols. All three are classified on the basis of their hydroxyl groups. Monohydric, Dihydric, and Trihydric alcohols contain one $ -OH $ group, two $ -OH $ groups, and three groups, respectively. Moreover, the other three types of alcohol are primary, secondary, and tertiary alcohols. This reaction takes place in the presence of protic acid i.e. sulphuric acid alcohol undergoes dehydration to produce alkenes and ether as their minor and major products. This reaction occurs. This is the ideal method of preparation of ether.
The reaction of $ RCH=C{{H}_{2}} $ with $ B{{H}_{3}}THF $ followed by oxidation with alkaline $ {{H}_{2}}{{O}_{2}} $ gives $ RC{{H}_{2}}C{{H}_{2}}OH. $
$ RCH=C{{H}_{2}}\xrightarrow[C{{H}_{2}}COOH]{B{{H}_{3}}THF}\underset{\left( A \right)}{\mathop{{{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B}}\,\xrightarrow[{}]{{{H}_{2}}{{O}_{2}}/O{{H}^{-}}}\underset{\left( B \right)}{\mathop{RC{{H}_{2}}C{{H}_{2}}OH}}\, $
$ \underset{\left( B \right)}{\mathop{RC{{H}_{2}}C{{H}_{2}}OH}}\,\xrightarrow{\underset{\left( D \right)}{\mathop{HI}}\,}\underset{\left( C \right)}{\mathop{RC{{H}_{2}}C{{H}_{3}}}}\, $
Therefore, sequence of reaction A, B, C and D are respectively are $ \left[ {{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B \right],\left[ RC{{H}_{2}}C{{H}_{2}}OH \right],\left[ RC{{H}_{2}}C{{H}_{3}} \right],\left[ HI \right] $ .
Note :
Remember that the monohydric phenol contains one alcoholic group. The dihydric phenols have two $ -OH $ groups. They can be ortho, Meta, and even para-derivative. Finally, the third type of phenols is trihydric phenols, which have three alcoholic groups. When it comes to the classification of ether, students will learn about two types of ether; the first one is symmetrical ether, which is also known as simple ether; in this form of ether, the alkyl or the aryl groups attached to either side of the oxygen atoms are the same.
Complete Step By Step Answer:
When hydroxyl group atoms form a bond with saturated carbon atoms, alcohol is formed. Now, if the dehydration of alcohol takes place, then we get ether. In this chapter, you will get to learn how these compounds are connected to one another. In addition to this, there are three types of alcohols: Monohydric, Dihydric, and Trihydric alcohols. All three are classified on the basis of their hydroxyl groups. Monohydric, Dihydric, and Trihydric alcohols contain one $ -OH $ group, two $ -OH $ groups, and three groups, respectively. Moreover, the other three types of alcohol are primary, secondary, and tertiary alcohols. This reaction takes place in the presence of protic acid i.e. sulphuric acid alcohol undergoes dehydration to produce alkenes and ether as their minor and major products. This reaction occurs. This is the ideal method of preparation of ether.
The reaction of $ RCH=C{{H}_{2}} $ with $ B{{H}_{3}}THF $ followed by oxidation with alkaline $ {{H}_{2}}{{O}_{2}} $ gives $ RC{{H}_{2}}C{{H}_{2}}OH. $
$ RCH=C{{H}_{2}}\xrightarrow[C{{H}_{2}}COOH]{B{{H}_{3}}THF}\underset{\left( A \right)}{\mathop{{{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B}}\,\xrightarrow[{}]{{{H}_{2}}{{O}_{2}}/O{{H}^{-}}}\underset{\left( B \right)}{\mathop{RC{{H}_{2}}C{{H}_{2}}OH}}\, $
$ \underset{\left( B \right)}{\mathop{RC{{H}_{2}}C{{H}_{2}}OH}}\,\xrightarrow{\underset{\left( D \right)}{\mathop{HI}}\,}\underset{\left( C \right)}{\mathop{RC{{H}_{2}}C{{H}_{3}}}}\, $
Therefore, sequence of reaction A, B, C and D are respectively are $ \left[ {{\left( RC{{H}_{2}}C{{H}_{2}} \right)}_{3}}B \right],\left[ RC{{H}_{2}}C{{H}_{2}}OH \right],\left[ RC{{H}_{2}}C{{H}_{3}} \right],\left[ HI \right] $ .
Note :
Remember that the monohydric phenol contains one alcoholic group. The dihydric phenols have two $ -OH $ groups. They can be ortho, Meta, and even para-derivative. Finally, the third type of phenols is trihydric phenols, which have three alcoholic groups. When it comes to the classification of ether, students will learn about two types of ether; the first one is symmetrical ether, which is also known as simple ether; in this form of ether, the alkyl or the aryl groups attached to either side of the oxygen atoms are the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




