
In the structure of diborane,
A. All hydrogen atoms lie in one plane and born atoms lie in a plane perpendicular to this plane.
B. 2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 4 bridged hydrogens atoms lie in the perpendicular plane
C. 4 bridging hydrogens atoms and boron atoms lie in one plane and 2 terminal hydrogen atoms lie in a plane perpendicular to this plane
D. All the atoms are in the same plane.
Answer
572.1k+ views
Hint: The molecular formula of diborane is ${{B}_{2}}{{H}_{6}}$ . Diborane has two boron atoms and six hydrogen atoms in its structure. In the structure of diborane there are four terminal hydrogens and two bridged hydrogens.
Complete Solution :
- In the question it is asked the position of the hydrogens and position of the boron atom in the structure of the diborane.
- To know about the position of the 6 hydrogens and 2 boron atoms we should draw the structure of the diborane.
- The structure of the diborane is as follows.
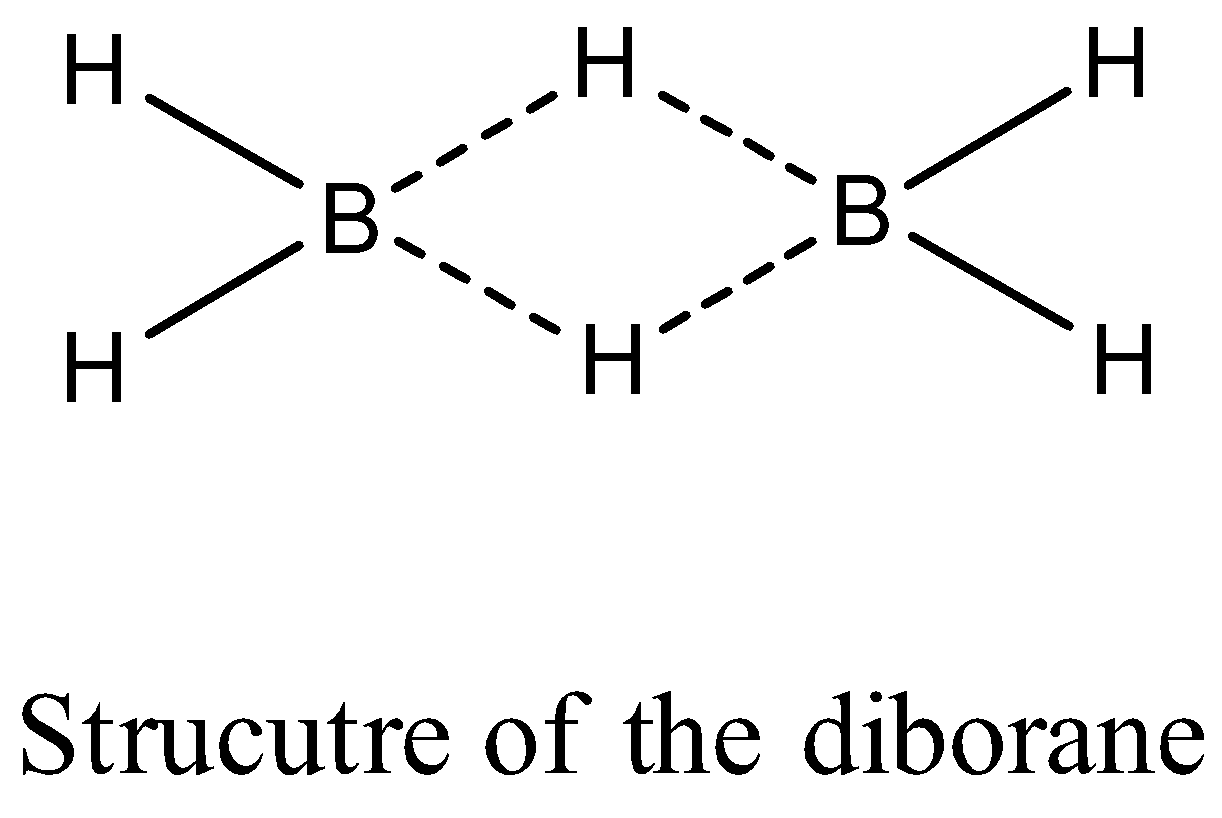
- In the structure of the diborane we can see clearly that there are two types of hydrogens.
- Four hydrogens are of one type and called terminal hydrogens and two hydrogens are of one type and called bridged hydrogens.
- The four hydrogen atoms and two born atoms lie in one plane and the bridged hydrogen atoms lie in different planes.
So, the correct answer is “Option C”.
Note: The bridged hydrogen atoms are perpendicular to the plane which contains 4 terminal hydrogen atoms and two boron atoms. The two hydrogen atoms which hold the born atoms together are called bridged hydrogens.
Complete Solution :
- In the question it is asked the position of the hydrogens and position of the boron atom in the structure of the diborane.
- To know about the position of the 6 hydrogens and 2 boron atoms we should draw the structure of the diborane.
- The structure of the diborane is as follows.

- In the structure of the diborane we can see clearly that there are two types of hydrogens.
- Four hydrogens are of one type and called terminal hydrogens and two hydrogens are of one type and called bridged hydrogens.
- The four hydrogen atoms and two born atoms lie in one plane and the bridged hydrogen atoms lie in different planes.
So, the correct answer is “Option C”.
Note: The bridged hydrogen atoms are perpendicular to the plane which contains 4 terminal hydrogen atoms and two boron atoms. The two hydrogen atoms which hold the born atoms together are called bridged hydrogens.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




