
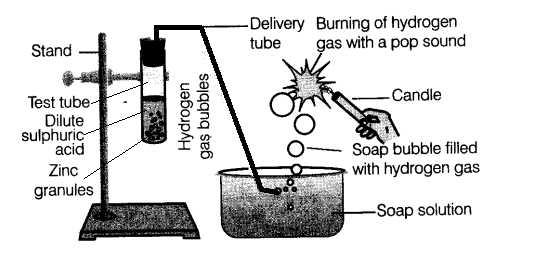
In the schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if the following changes are made?
In place of zinc, copper turnings are taken.
(A) More Hydrogen gas is evolved.
(B) Less Hydrogen gas is evolved.
(C) Almost an equal amount of gas is evolved.
(D) Hydrogen gas is not evolved.

Answer
568.8k+ views
Hint: Zinc lies above hydrogen whereas copper lies below hydrogen in the electrochemical series. Zn is more reactive than ${{H}^{+}}$ whereas Cu is less reactive.
Complete Solution :
Let us first know about the mechanism of the preparation of hydrogen gas to specifically tell what would happen if the reagent is changed.
The laboratory preparation of hydrogen gas:
In the laboratory the hydrogen gas is prepared with the help of dilute sulphuric acid or dilute hydrochloric acid and zinc granules.
Granulated zinc is ideally used for preparation of hydrogen gas in chemical laboratories. As, it usually contains a small amount of copper that acts as a catalyst to the connected chemical reaction. Therefore, this increases the rate of the chemical reaction without actually participating in the reaction.
Procedure-
Step 1-
Take a few grams of zinc granules and place them in a 500 ml flask.
Step 2-
With the help of a thistle funnel, add dilute hydrochloric acid or dilute sulphuric acid to the zinc granules. (Dilute sulphuric acid is the substitute of dilute hydrochloric acid in its absence).
Step 3-
Hydrogen gas will be collected with the help of a delivery tube through the downward displacement of water. (As hydrogen gas is lighter than water).
General format of reaction taking place-
Metal + Dilute acid $\to $ Salt of metal and acid + Hydrogen
Here, as two acids can be used:
With Hydrochloric Acid-
$Zn+2HCl\to ZnC{{l}_{2}}+{{H}_{2}}$
With Sulphuric Acid-
$Zn+{{H}_{2}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}$
Some precautions must be taken as hydrogen can explosively react with air in nature.
Illustration-
We have asked for the replacement of zinc granules with copper tunings.
Copper is less reactive, so it does not react with dilute sulphuric acid or dilute hydrochloric acid. Hence, no reaction will take place resulting in no evolution of hydrogen gas.
So, the correct answer is “Option D”.
Note: The hydrogen gas evolved depends on the reactive nature of the replacive element. Do check the reactivities of the element with dilute sulphuric acid and dilute hydrochloric acid.
Complete Solution :
Let us first know about the mechanism of the preparation of hydrogen gas to specifically tell what would happen if the reagent is changed.
The laboratory preparation of hydrogen gas:
In the laboratory the hydrogen gas is prepared with the help of dilute sulphuric acid or dilute hydrochloric acid and zinc granules.
Granulated zinc is ideally used for preparation of hydrogen gas in chemical laboratories. As, it usually contains a small amount of copper that acts as a catalyst to the connected chemical reaction. Therefore, this increases the rate of the chemical reaction without actually participating in the reaction.
Procedure-
Step 1-
Take a few grams of zinc granules and place them in a 500 ml flask.
Step 2-
With the help of a thistle funnel, add dilute hydrochloric acid or dilute sulphuric acid to the zinc granules. (Dilute sulphuric acid is the substitute of dilute hydrochloric acid in its absence).
Step 3-
Hydrogen gas will be collected with the help of a delivery tube through the downward displacement of water. (As hydrogen gas is lighter than water).
General format of reaction taking place-
Metal + Dilute acid $\to $ Salt of metal and acid + Hydrogen
Here, as two acids can be used:
With Hydrochloric Acid-
$Zn+2HCl\to ZnC{{l}_{2}}+{{H}_{2}}$
With Sulphuric Acid-
$Zn+{{H}_{2}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}$
Some precautions must be taken as hydrogen can explosively react with air in nature.
Illustration-
We have asked for the replacement of zinc granules with copper tunings.
Copper is less reactive, so it does not react with dilute sulphuric acid or dilute hydrochloric acid. Hence, no reaction will take place resulting in no evolution of hydrogen gas.
So, the correct answer is “Option D”.
Note: The hydrogen gas evolved depends on the reactive nature of the replacive element. Do check the reactivities of the element with dilute sulphuric acid and dilute hydrochloric acid.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




