
In the reaction, ${C_6}{H_5}N{H_2}\xrightarrow[{273K}]{{NaN{O_2}/HB{F_4}}}(A)\xrightarrow{{\Delta H}}{C_6}{H_5}F$ the compound A is known as:
A) m-nitro fluorobenzene
B) A mixture of Fluor anilines
C) Benzene Diazonium Fluoride
D) Benzene Diazonium Tetrafluoroborate
Answer
498.3k+ views
Hint: When aniline reacts with Sodium Nitrite and Hydrochloric acid at $0 - {5^ \circ }C$ to form Benzene Diazonium Salts is known as Diazotization. Benzene Diazonium Halides can be used as an intermediate to form a variety of Aryl Halides, Carboxylic Acids, etc.
Complete answer:
Aromatic Amines (aniline) react with Nitrous Acid (which is formed in the reaction on reaction with Sodium Nitrite and Mineral Acid) to form diazonium salts and water as a by-product. The diazotization reaction was first given by Peter Griess. The general form of the reaction can be shown as:
$ArN{H_2} + HN{O_2} + HX \to RN_2^ + {X^ - } + {H_2}O$
The reaction given to us is known as the Schiemann reaction (also called the Balz–Schiemann reaction). This reaction involves the conversion of Aryl Amines to Aryl Fluorides by forming a Diazonium Tetrafluoroborate intermediate. This reaction preferentially uses $HB{F_4}$ as a reagent/mineral acid. This reaction is very similar to Sandmeyer’s Reaction for the formation of Aryl Chloride and bromides, but the difference is the catalyst used. Sandmeyer’s reaction uses copper catalyst.
Many innovations have been made in the Schiemann reaction by using hexafluorophosphate and hexafluoro antimonates for better yield.
The complete reaction given to us can be shown as:
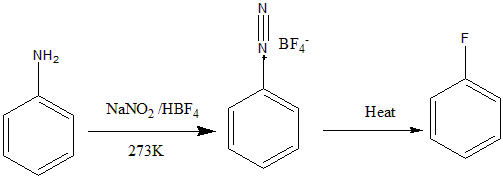
Therefore, the compound A formed is Benzene diazonium tetrafluoroborate.
The correct option is (D).
Note:
The conversion of Benzene Diazonium Fluoride to Aryl Fluoride proceeds without any promoter, with the formation of highly unstable $A{r^ + }$ which abstracts the fluoride from $HB{F_4}$ to give aryl fluoride (ArF). Boron Trifluoride is formed as a by-product. Schiemann reaction is a traditional reaction for the formation of Aryl Fluorides along with some derivatives like 4-fluoro benzoic acid.
Complete answer:
Aromatic Amines (aniline) react with Nitrous Acid (which is formed in the reaction on reaction with Sodium Nitrite and Mineral Acid) to form diazonium salts and water as a by-product. The diazotization reaction was first given by Peter Griess. The general form of the reaction can be shown as:
$ArN{H_2} + HN{O_2} + HX \to RN_2^ + {X^ - } + {H_2}O$
The reaction given to us is known as the Schiemann reaction (also called the Balz–Schiemann reaction). This reaction involves the conversion of Aryl Amines to Aryl Fluorides by forming a Diazonium Tetrafluoroborate intermediate. This reaction preferentially uses $HB{F_4}$ as a reagent/mineral acid. This reaction is very similar to Sandmeyer’s Reaction for the formation of Aryl Chloride and bromides, but the difference is the catalyst used. Sandmeyer’s reaction uses copper catalyst.
Many innovations have been made in the Schiemann reaction by using hexafluorophosphate and hexafluoro antimonates for better yield.
The complete reaction given to us can be shown as:

Therefore, the compound A formed is Benzene diazonium tetrafluoroborate.
The correct option is (D).
Note:
The conversion of Benzene Diazonium Fluoride to Aryl Fluoride proceeds without any promoter, with the formation of highly unstable $A{r^ + }$ which abstracts the fluoride from $HB{F_4}$ to give aryl fluoride (ArF). Boron Trifluoride is formed as a by-product. Schiemann reaction is a traditional reaction for the formation of Aryl Fluorides along with some derivatives like 4-fluoro benzoic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




