
In the reaction ${C_6}{H_5}COC{H_3}\xrightarrow[{Zn - Hg/conc.HCl}]{{[H]}}X$. X is:
A.Toluene
B.Methylbenzene
C.Benzyl Alcohol
D.Ethylbenzene
Answer
585k+ views
Hint: The reduction of the aldehydes and ketones to the hydrocarbons in the presence of the zinc amalgam (Zn/ Hg) and concentrated HCl is known as Clemmensen Reduction. This reaction is of main use in the reduction process of the aldehydes and ketones to get the higher order hydrocarbons.
Complete step by step answer:
The deoxygenation of the corresponding aldehydes and ketones takes place to produce the hydrocarbons, i.e. the compounds consisting mainly of carbon and hydrogen. The catalyst used in the process is the Zinc Amalgam. The compounds of mercury with the metals are known as amalgams, they are given by the general formula M-Hg where M is the metal.
Every reaction follows a particular reaction mechanism the general mechanism for the Clemmensen reduction is as follows:
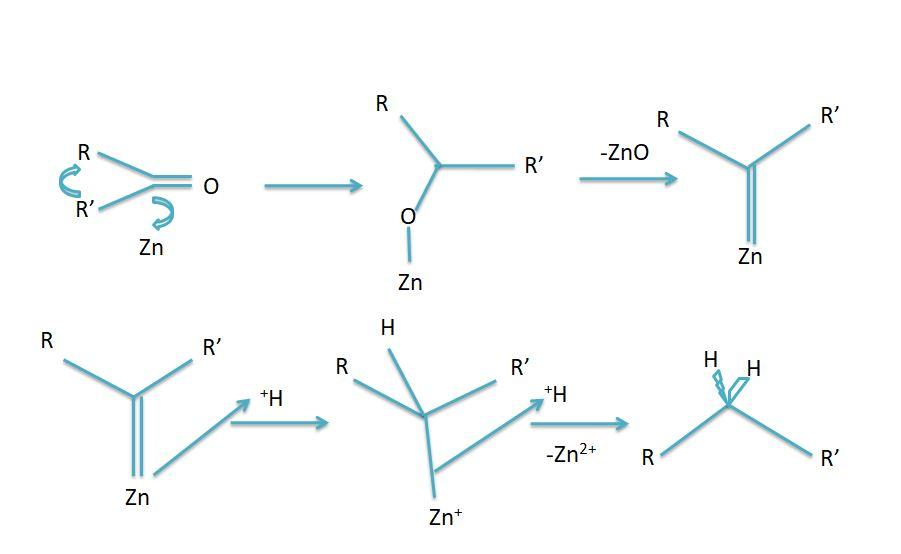
However the mechanism is not completely understood yet but two mechanisms were put forward to know the probable mechanism of the reaction these are:
-Carbenoid Mechanism: This mechanism states that the reduction process takes place on the surface of the zinc catalyst. No alcohol as an intermediate is formed in the reaction.
-Carbanionic mechanism: This mechanism states that zinc metal directly attacks the protonated carbon.

Thus in the above reaction Methylbenzene will be formed as follows:
Another complementary reaction used for such purpose to obtain the hydrocarbon is the Wolff-Kishner Reduction, in which the reaction condition is strongly basic whereas acidic conditions are used in the Clemmensen reaction; the substrate must be stable to acid to undergo the Clemmensen reaction.
So the option B is the correct answer.
Note:
The Clemmensen reduction reaction is more effective for the cyclic ketones or aliphatic compounds and for the zinc metal i.e. for the reduction of the aryl-alkyl ketones which are actively formed during the Friedel-Crafts acylation reaction.
Complete step by step answer:
The deoxygenation of the corresponding aldehydes and ketones takes place to produce the hydrocarbons, i.e. the compounds consisting mainly of carbon and hydrogen. The catalyst used in the process is the Zinc Amalgam. The compounds of mercury with the metals are known as amalgams, they are given by the general formula M-Hg where M is the metal.
Every reaction follows a particular reaction mechanism the general mechanism for the Clemmensen reduction is as follows:

However the mechanism is not completely understood yet but two mechanisms were put forward to know the probable mechanism of the reaction these are:
-Carbenoid Mechanism: This mechanism states that the reduction process takes place on the surface of the zinc catalyst. No alcohol as an intermediate is formed in the reaction.
-Carbanionic mechanism: This mechanism states that zinc metal directly attacks the protonated carbon.

Thus in the above reaction Methylbenzene will be formed as follows:
Another complementary reaction used for such purpose to obtain the hydrocarbon is the Wolff-Kishner Reduction, in which the reaction condition is strongly basic whereas acidic conditions are used in the Clemmensen reaction; the substrate must be stable to acid to undergo the Clemmensen reaction.
So the option B is the correct answer.
Note:
The Clemmensen reduction reaction is more effective for the cyclic ketones or aliphatic compounds and for the zinc metal i.e. for the reduction of the aryl-alkyl ketones which are actively formed during the Friedel-Crafts acylation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




