
In the molecule $ {H_2}S $ , four electron groups around the sulphur atom are arranged in a tetrahedral geometry, but the shape of the molecule is called "bent." Why does the shape have a different name from the name of the electron group geometry?
Answer
490.8k+ views
Hint: We can know the hybridisation of any molecule using this simple formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer we get is:
Complete answer:
First we will understand two terms, molecular geometry (also known as the shape of the molecule) and electron pair geometry. The molecular geometry deals with the arrangements of all the atoms that make up the molecule and the electron pair geometry is the arrangement of electron density that surrounds the molecule. The electron density can either be of a single, double or triple bond or a lone pair of electrons.
The electron pair geometry includes all the bond pairs as well as Lone pairs whereas the molecular geometry includes just the bonds. According to the formula given above, the hybridization of $ {H_2}S $ can be given as:
$ Hybridisation = \dfrac{1}{2}(6 + 2) = 4 \to s{p^3} $
The Lewis dot structure of $ {H_2}S $ is shown below.
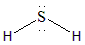
The Sulphur atom is surrounded by two hydrogen atoms and two lone pairs. This means that it has a steric number of 4. Hence the electron pair geometry with Steric Number 4 will be tetrahedral.
The coordination number of the molecule $ {H_2}S $ is 2. This only includes only the bonds of two hydrogen atoms. According to VSEPR theory, the molecular geometry for a molecule with a central atom surrounded by hydrogen atoms is Bent shape. The shape of molecule $ {H_2}O $ is similar to that of $ {H_2}S $ .
Note:
The electron pair geometry is the actual geometry of the molecule which includes the lone pairs also and can be different from the molecular geometry which is the shape of the molecule because it only considers the bonds to other atoms.
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer we get is:
| Answer Obtained/Steric Number | Hybridisation |
| 2 | sp |
| 3 | $ s{p^2} $ |
| 4 | $ s{p^3} $ |
| 5 | $ s{p^3}d $ |
| 6 | $ s{p^3}{d^2} $ |
Complete answer:
First we will understand two terms, molecular geometry (also known as the shape of the molecule) and electron pair geometry. The molecular geometry deals with the arrangements of all the atoms that make up the molecule and the electron pair geometry is the arrangement of electron density that surrounds the molecule. The electron density can either be of a single, double or triple bond or a lone pair of electrons.
The electron pair geometry includes all the bond pairs as well as Lone pairs whereas the molecular geometry includes just the bonds. According to the formula given above, the hybridization of $ {H_2}S $ can be given as:
$ Hybridisation = \dfrac{1}{2}(6 + 2) = 4 \to s{p^3} $
The Lewis dot structure of $ {H_2}S $ is shown below.
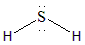
The Sulphur atom is surrounded by two hydrogen atoms and two lone pairs. This means that it has a steric number of 4. Hence the electron pair geometry with Steric Number 4 will be tetrahedral.
The coordination number of the molecule $ {H_2}S $ is 2. This only includes only the bonds of two hydrogen atoms. According to VSEPR theory, the molecular geometry for a molecule with a central atom surrounded by hydrogen atoms is Bent shape. The shape of molecule $ {H_2}O $ is similar to that of $ {H_2}S $ .
Note:
The electron pair geometry is the actual geometry of the molecule which includes the lone pairs also and can be different from the molecular geometry which is the shape of the molecule because it only considers the bonds to other atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




