
In the long form of periodic table, the total number of period is:
A.5
B.7
C.8
D.9
Answer
582.6k+ views
Hint: A periodic table consists of chemical elements that are arranged in the order of their atomic number. The periodic table is divided into groups and periods. The properties of the elements vary in groups and periods. The elements in a periodic table are arranged by atomic number, electronic configuration, and repeating chemical properties.
Complete step by step answer:
Now let us understand the group and period.
We know that the horizontal rows that go from left to right in a periodic table are called the period.
The vertical columns that go from top to bottom are called groups.
In a periodic table, there are 7 periods. Each period starts from the leftmost side to the right.
So, the correct answer is Option B.
Additional Information:
The number of groups in a periodic table is 18. Group 1, 2 and 13 to 18 are the main groups that are present. The elements in these groups are the main group elements. Every element in a group has the same number of electrons in the valence shell but they are present in different shells. For example, let us take Boron and Aluminium with atomic numbers 5 and 13 respectively. The valence electrons in both boron and aluminum are 3 but they are present in the L and M shell respectively.
Note:
All elements that are present in a row or a period have the same number of shells. For example, let us look at sodium and magnesium which is present in the third period of the periodic table.
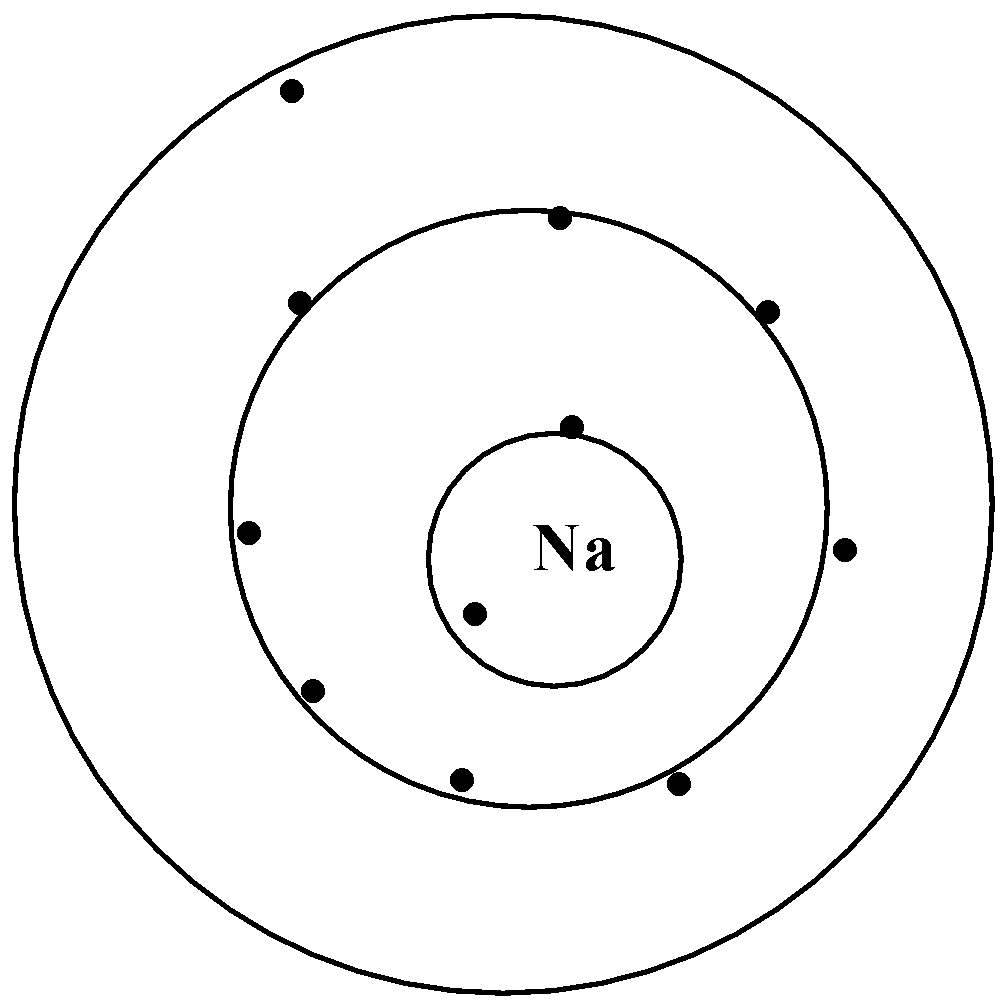
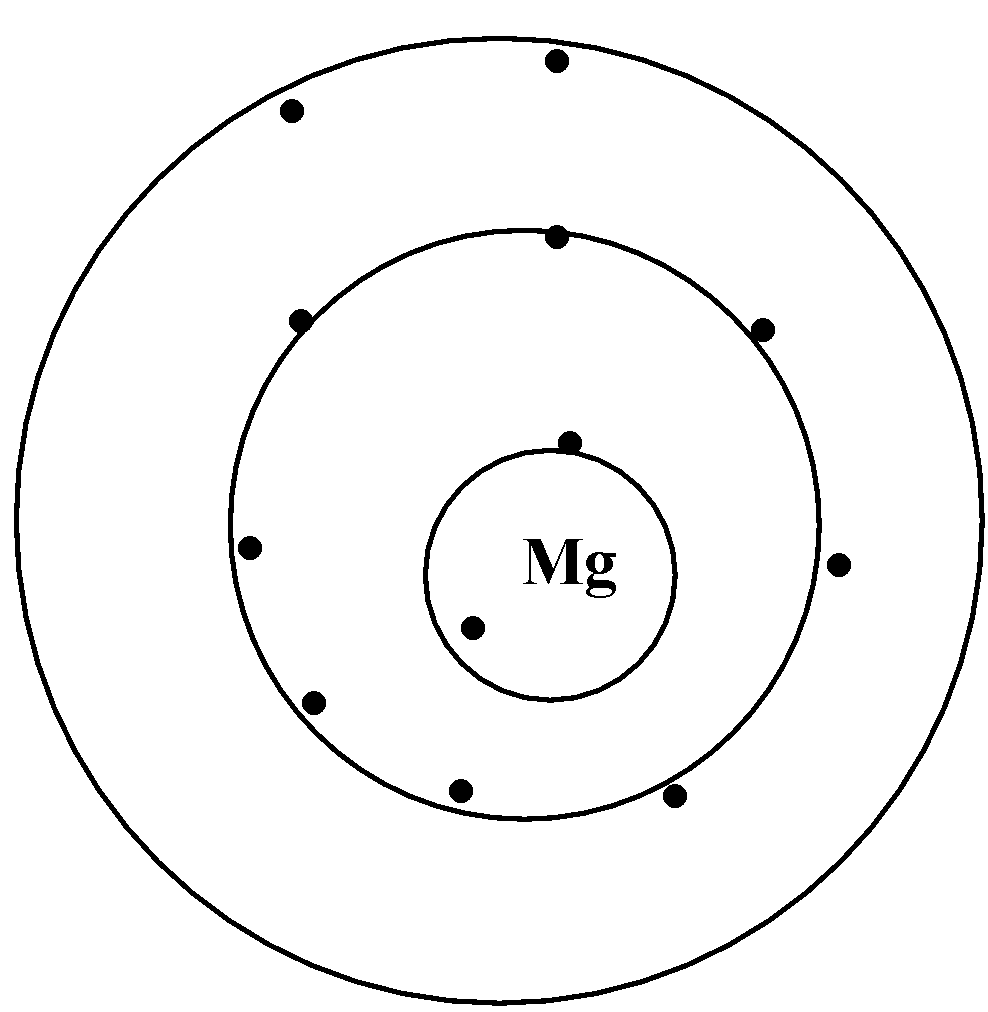
We can say that sodium and magnesium have the same number of shells but a different atomic number. On moving across a period from left to right, the atomic number increases, atomic size varies, electronegativity increases.
Complete step by step answer:
Now let us understand the group and period.
We know that the horizontal rows that go from left to right in a periodic table are called the period.
The vertical columns that go from top to bottom are called groups.
In a periodic table, there are 7 periods. Each period starts from the leftmost side to the right.
So, the correct answer is Option B.
Additional Information:
The number of groups in a periodic table is 18. Group 1, 2 and 13 to 18 are the main groups that are present. The elements in these groups are the main group elements. Every element in a group has the same number of electrons in the valence shell but they are present in different shells. For example, let us take Boron and Aluminium with atomic numbers 5 and 13 respectively. The valence electrons in both boron and aluminum are 3 but they are present in the L and M shell respectively.
Note:
All elements that are present in a row or a period have the same number of shells. For example, let us look at sodium and magnesium which is present in the third period of the periodic table.


We can say that sodium and magnesium have the same number of shells but a different atomic number. On moving across a period from left to right, the atomic number increases, atomic size varies, electronegativity increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




