
In the Lewis structure for the $O{{F}_{2}}$ molecule, the number of lone pairs of electrons around the central oxygen atom is:
(A)- 1
(B)- 2
(C)- 3
(D)- 4
Answer
584.4k+ views
Hint: The valence electrons remaining in the molecule, after the bond pairs have formed are the non-bonding electrons called the lone pair. In the central atom the lone pair along with the shard bond pair complete the octet.
Complete step by step answer:
- In the Lewis structure of $O{{F}_{2}}$, we have the oxygen being less electronegative than the fluorine atom. Thus, acting as the central atom in the structure.
- The oxygen atom has six valence electrons with outer shell configuration $2{{s}^{2}}2{{p}^{4}}$ and fluorine has seven valence electrons with outer shell configuration $2{{s}^{2}}2{{p}^{5}}$. So, the total number of valence electrons in the molecule from the oxygen atom and the two fluorine atoms attached to it is $(6+7+7=)\,20$ electrons.
- Each of these atoms follows octet rule and tries to attain eight electrons in its outer shell having p-orbital by sharing electrons with the adjacent atoms forming a bond. So, the oxygen shares two of its electrons with the two fluorine atoms, one on each side. Then, out of the 20 electrons, four electrons are used up in the forming the two $O-F$ single-bonds.
The remaining 16 electrons form the non-bonding lone pairs of electrons. The fluorine having three lone-pairs $\text{(3}\,\text{ }\!\!\times\!\!\text{ }\,\text{2}\,\text{=}\,\text{6}\,\,\text{electrons)}$ on each atom and the rest four electrons on oxygen as two lone-pairs. Thus, completing their octet.
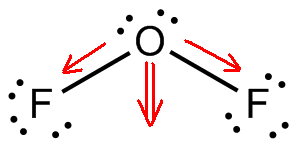
Therefore, from the Lewis structure it seen that in the central oxygen atom the number of lone pairs
So, the correct answer is “Option B”.
Note: This molecule is bent- shaped due to the two lone pairs of electrons on central atom, thereby preventing the cancellation of the charge on the adjacent fluorine atoms, which further attracts the bonding electrons towards itself due to higher electronegativity. Thus, it has a small dipole moment and forms polar molecules.
Complete step by step answer:
- In the Lewis structure of $O{{F}_{2}}$, we have the oxygen being less electronegative than the fluorine atom. Thus, acting as the central atom in the structure.
- The oxygen atom has six valence electrons with outer shell configuration $2{{s}^{2}}2{{p}^{4}}$ and fluorine has seven valence electrons with outer shell configuration $2{{s}^{2}}2{{p}^{5}}$. So, the total number of valence electrons in the molecule from the oxygen atom and the two fluorine atoms attached to it is $(6+7+7=)\,20$ electrons.
- Each of these atoms follows octet rule and tries to attain eight electrons in its outer shell having p-orbital by sharing electrons with the adjacent atoms forming a bond. So, the oxygen shares two of its electrons with the two fluorine atoms, one on each side. Then, out of the 20 electrons, four electrons are used up in the forming the two $O-F$ single-bonds.
The remaining 16 electrons form the non-bonding lone pairs of electrons. The fluorine having three lone-pairs $\text{(3}\,\text{ }\!\!\times\!\!\text{ }\,\text{2}\,\text{=}\,\text{6}\,\,\text{electrons)}$ on each atom and the rest four electrons on oxygen as two lone-pairs. Thus, completing their octet.

Therefore, from the Lewis structure it seen that in the central oxygen atom the number of lone pairs
So, the correct answer is “Option B”.
Note: This molecule is bent- shaped due to the two lone pairs of electrons on central atom, thereby preventing the cancellation of the charge on the adjacent fluorine atoms, which further attracts the bonding electrons towards itself due to higher electronegativity. Thus, it has a small dipole moment and forms polar molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




