
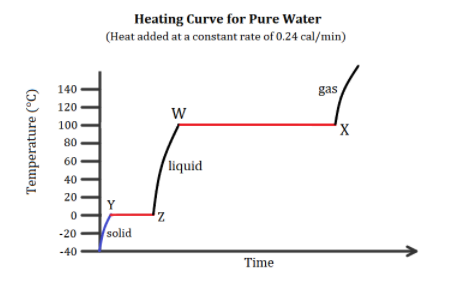
In the heating curve of water shown below, why is line \[WX\] longer than line \[YZ\]?

Answer
524.4k+ views
Hint: The above graph describes the change of phase of water from its solid form to liquid form and then to its gaseous form. Check the temperature and time required by each line to better understand how heat corresponds to the phase changes from ice to water and then to its vapour form. Then compare the molar enthalpy of both the phase change to explain the given question.
Complete step-by-step answer:
The above heating curve of water shows that water is first converted from its solid form to its liquid form by melting it. Then the liquid form is boiled to the gaseous form. The \[YZ\] line is at a constant temperature of \[0^\circ C\] which is also the melting point of ice. Hence, there is a melting phase transition of ice by heating it over time.
The \[WX\] line is at a constant temperature of \[100^\circ C\] which is also the boiling point of water. Hence, there is again a phase transition of water by boiling it over time. The molar enthalpy of ice at \[0^\circ C\] or \[273K\], \[\Delta {{\rm H}_{fusion}}\] is \[6.02kJ/mol\]at normal atmospheric pressure. Similarly, the molar enthalpy of vaporizing for water, \[\Delta {{\rm H}_{vaporisation}}\] is \[40.7kJ/mol\].
Here, the enthalpy of vaporizing for water is larger as compared to the enthalpy of fusion. This larger enthalpy indicates a larger amount of heat being applied at constant pressure in order to perform the phase transition. Since more heat is required to vaporize the water, hence, more time is required at the same constant heating rate to vaporize water. Thus, \[WX\] line is longer than \[YZ\] line.
Note: The value of molar enthalpy of ice has a constant value of \[6.02kJ/mol\] only at \[273K\] and at normal pressure. Similarly, the molar enthalpy of vaporization for water has a constant value of \[40.7kJ/mol\] at \[373K\] and at normal pressure. Any change in these conditions will also alter the values of enthalpy.
Complete step-by-step answer:
The above heating curve of water shows that water is first converted from its solid form to its liquid form by melting it. Then the liquid form is boiled to the gaseous form. The \[YZ\] line is at a constant temperature of \[0^\circ C\] which is also the melting point of ice. Hence, there is a melting phase transition of ice by heating it over time.
The \[WX\] line is at a constant temperature of \[100^\circ C\] which is also the boiling point of water. Hence, there is again a phase transition of water by boiling it over time. The molar enthalpy of ice at \[0^\circ C\] or \[273K\], \[\Delta {{\rm H}_{fusion}}\] is \[6.02kJ/mol\]at normal atmospheric pressure. Similarly, the molar enthalpy of vaporizing for water, \[\Delta {{\rm H}_{vaporisation}}\] is \[40.7kJ/mol\].
Here, the enthalpy of vaporizing for water is larger as compared to the enthalpy of fusion. This larger enthalpy indicates a larger amount of heat being applied at constant pressure in order to perform the phase transition. Since more heat is required to vaporize the water, hence, more time is required at the same constant heating rate to vaporize water. Thus, \[WX\] line is longer than \[YZ\] line.
Note: The value of molar enthalpy of ice has a constant value of \[6.02kJ/mol\] only at \[273K\] and at normal pressure. Similarly, the molar enthalpy of vaporization for water has a constant value of \[40.7kJ/mol\] at \[373K\] and at normal pressure. Any change in these conditions will also alter the values of enthalpy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




