
In the HCl molecule, the separation between the nuclei of two atoms is about \[1.27\,\mathop {\text{A}}\limits^{\text{o}} \](\[1\,\mathop {\text{A}}\limits^{\text{o}} = {10^{ - 10}}\,{\text{m}}\]). The approximate location of the center of mass of the molecule, assuming the chlorine atom to be about 35.5 times massive as hydrogen is
A. \[1\,\mathop {\text{A}}\limits^{\text{o}} \]
B. \[1.5\,\mathop {\text{A}}\limits^{\text{o}} \]
C. \[1.24\,\mathop {\text{A}}\limits^{\text{o}} \]
D. \[2.5\,\mathop {\text{A}}\limits^{\text{o}} \]
Answer
527k+ views
Hint: Use the formula for the position of the center of mass of two objects. Assume that the center of mass is at the origin and hydrogen and chlorine atoms are on either sides of the origin.
Formula used:
The position \[{x_{CM}}\] of the center of mass of two objects from the origin is
\[{x_{CM}} = \dfrac{{{m_1}{x_1} + {m_2}{x_2}}}{{{m_1} + {m_2}}}\] …… (1)
Here, \[{m_1}\] and \[{m_2}\] are the masses of two objects and \[{x_1}\] and \[{x_2}\]are the positions of the two objects of masses \[{m_1}\] and \[{m_2}\]from the origin respectively.
Complete step by step answer:
In the HCl molecule, the separation between the nuclei of two atoms is about \[1.27\,\mathop {\text{A}}\limits^{\text{o}} \].
Let \[{m_H}\] be the mass of the hydrogen atom and \[{m_{Cl}}\] be the mass of the chlorine atom.
The mass of the chlorine atom is 35.5 times more than the hydrogen atom.
\[{m_{Cl}} = 35.5{m_H}\]
The diagram representing the position of center of mass of HCl molecule is as follows:
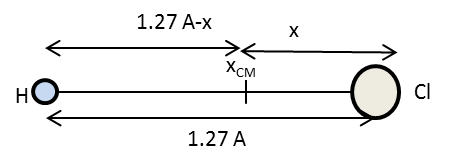
In the above diagram, \[x\] is the distance of chlorine atom from the origin and \[1.27\,\mathop {\text{A}}\limits^{\text{o}} - x\] is the distance of hydrogen atom from the origin.
Let the center of mass of HCl molecules is at the origin.
Determine the position of the center of mass of HCl molecules.
Substitute \[0\] for \[{x_{CM}}\], \[{m_{Cl}}\] for \[{m_1}\], \[{m_H}\] for \[{m_2}\], \[x\] for \[{x_1}\] and \[ - \left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)\] for \[{x_2}\] in equation (1).
\[0 = \dfrac{{{m_{Cl}}x + \left[ {{m_H} - \left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)} \right]}}{{{m_{Cl}} + {m_H}}}\]
\[0 = \dfrac{{{m_{Cl}}x - {m_H}\left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)}}{{{m_{Cl}} + {m_H}}}\]
Substitute \[35.5{m_H}\] for \[{m_{Cl}}\] in the above equation.
\[0 = \dfrac{{35.5{m_H}x - {m_H}\left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)}}{{35.5{m_H} + {m_H}}}\]
\[ \Rightarrow 35.5{m_H}x - {m_H}1.27\,\mathop {\text{A}}\limits^{\text{o}} + {m_H}x = 0\]
\[ \Rightarrow 35.5x - 1.27\,\mathop {\text{A}}\limits^{\text{o}} + x = 0\]
\[ \Rightarrow 36.5x - 1.27\,\mathop {\text{A}}\limits^{\text{o}} = 0\]
\[ \Rightarrow x = \dfrac{{1.27\,\mathop {\text{A}}\limits^{\text{o}} }}{{36.5}}\]
\[ \Rightarrow x = 0.0347\,\mathop {\text{A}}\limits^{\text{o}} \]
\[ \Rightarrow x \approx 0.035\,\mathop {\text{A}}\limits^{\text{o}} \]
Hence, the location of the center of mass of HCl molecules is \[0.035\,\mathop {\text{A}}\limits^{\text{o}} \] from the chlorine atom.
The position of the center of mass from hydrogen atom is
\[1.27\,\mathop {\text{A}}\limits^{\text{o}} - 0.035\,\mathop {\text{A}}\limits^{\text{o}} = 1.235\,\mathop {\text{A}}\limits^{\text{o}} \]
Therefore, the approximate position of the center of mass from the hydrogen atom is \[1.24\,\mathop {\text{A}}\limits^{\text{o}} \].
So, the correct answer is “Option C”.
Note:
The distance of hydrogen atom from the center of mass is taken negative as the hydrogen atom is on the left side of the origin (center of mass).
The center of mass is at the origin and hydrogen and chlorine atoms are on either sides of the origin.
Formula used:
The position \[{x_{CM}}\] of the center of mass of two objects from the origin is
\[{x_{CM}} = \dfrac{{{m_1}{x_1} + {m_2}{x_2}}}{{{m_1} + {m_2}}}\] …… (1)
Here, \[{m_1}\] and \[{m_2}\] are the masses of two objects and \[{x_1}\] and \[{x_2}\]are the positions of the two objects of masses \[{m_1}\] and \[{m_2}\]from the origin respectively.
Complete step by step answer:
In the HCl molecule, the separation between the nuclei of two atoms is about \[1.27\,\mathop {\text{A}}\limits^{\text{o}} \].
Let \[{m_H}\] be the mass of the hydrogen atom and \[{m_{Cl}}\] be the mass of the chlorine atom.
The mass of the chlorine atom is 35.5 times more than the hydrogen atom.
\[{m_{Cl}} = 35.5{m_H}\]
The diagram representing the position of center of mass of HCl molecule is as follows:

In the above diagram, \[x\] is the distance of chlorine atom from the origin and \[1.27\,\mathop {\text{A}}\limits^{\text{o}} - x\] is the distance of hydrogen atom from the origin.
Let the center of mass of HCl molecules is at the origin.
Determine the position of the center of mass of HCl molecules.
Substitute \[0\] for \[{x_{CM}}\], \[{m_{Cl}}\] for \[{m_1}\], \[{m_H}\] for \[{m_2}\], \[x\] for \[{x_1}\] and \[ - \left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)\] for \[{x_2}\] in equation (1).
\[0 = \dfrac{{{m_{Cl}}x + \left[ {{m_H} - \left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)} \right]}}{{{m_{Cl}} + {m_H}}}\]
\[0 = \dfrac{{{m_{Cl}}x - {m_H}\left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)}}{{{m_{Cl}} + {m_H}}}\]
Substitute \[35.5{m_H}\] for \[{m_{Cl}}\] in the above equation.
\[0 = \dfrac{{35.5{m_H}x - {m_H}\left( {1.27\,\mathop {\text{A}}\limits^{\text{o}} - x} \right)}}{{35.5{m_H} + {m_H}}}\]
\[ \Rightarrow 35.5{m_H}x - {m_H}1.27\,\mathop {\text{A}}\limits^{\text{o}} + {m_H}x = 0\]
\[ \Rightarrow 35.5x - 1.27\,\mathop {\text{A}}\limits^{\text{o}} + x = 0\]
\[ \Rightarrow 36.5x - 1.27\,\mathop {\text{A}}\limits^{\text{o}} = 0\]
\[ \Rightarrow x = \dfrac{{1.27\,\mathop {\text{A}}\limits^{\text{o}} }}{{36.5}}\]
\[ \Rightarrow x = 0.0347\,\mathop {\text{A}}\limits^{\text{o}} \]
\[ \Rightarrow x \approx 0.035\,\mathop {\text{A}}\limits^{\text{o}} \]
Hence, the location of the center of mass of HCl molecules is \[0.035\,\mathop {\text{A}}\limits^{\text{o}} \] from the chlorine atom.
The position of the center of mass from hydrogen atom is
\[1.27\,\mathop {\text{A}}\limits^{\text{o}} - 0.035\,\mathop {\text{A}}\limits^{\text{o}} = 1.235\,\mathop {\text{A}}\limits^{\text{o}} \]
Therefore, the approximate position of the center of mass from the hydrogen atom is \[1.24\,\mathop {\text{A}}\limits^{\text{o}} \].
So, the correct answer is “Option C”.
Note:
The distance of hydrogen atom from the center of mass is taken negative as the hydrogen atom is on the left side of the origin (center of mass).
The center of mass is at the origin and hydrogen and chlorine atoms are on either sides of the origin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




