
In the given reactions, the rate of reaction of (I) and (II) are the same. Both reactions proceed by which mechanism?
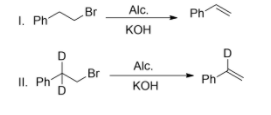
(A)- ${{E}_{1}}$
(B)- ${{E}_{2}}$
(C)- ${{E}_{1}}cb$
(D)- Anti-elimination

Answer
585.9k+ views
Hint: The type of organic reaction in which two substituents are removed from a molecule in either a one-step mechanism or two-step mechanism is known as an elimination reaction. $\beta $-elimination is a chemical reaction in which atoms or groups are lost from adjacent atoms resulting in a new pi-bond formation, usually between two carbon atoms. The atoms lost is usually a proton, but not always.
Complete Step by step solution:
-When the reaction follows a one-step mechanism for elimination reaction, such reactions are said to be ${{E}_{2}}$reaction. When the reaction follows a two-step mechanism for elimination reaction, such reactions are said to be ${{E}_{1}}$reactions.
-The number 1 and 2 in ${{E}_{1}}$and ${{E}_{2}}$does not refers to the number of steps in the mechanisms rather refers to the kinetics of the reaction, that is ${{E}_{2}}$is a bimolecular (second-order) reaction while ${{E}_{1}}$is a unimolecular (first-order) reaction.
-${{E}_{1}}cb$ exists where the molecule can stabilize an anion but possesses a poor leaving group.
-Following the ${{E}_{1}}$mechanism, carbocation is formed from the heterolytic cleavage of the C-Br bond. This is a slow step, hence is the rate-determining step. The proton abstraction now occurs from the adjacent carbon resulting in the formation of an alkene. Thus, only halide affects the rate of reaction.
-Following ${{E}_{2}}$mechanism, both Br atom and Deuterium or Hydrogen atom leave simultaneously and thus affect the rate.
-Following ${{E}_{1}}cb$, the first step is the removal of proton resulting in carbanion formation followed by the cleavage of halide or leaving group. Since, ${{E}_{1}}cb$ is a two-step mechanism, an isotope effect will be present.
-Anti-elimination and ${{E}_{2}}$eliminations are the same.
Therefore the correct answer is option A.
Note: The reactivity of halogens, iodide, and bromine are being favoured for influencing the rate of reaction. Fluoride not being a good leaving group hence gives elimination reactions at slower rates than other halogens. There is a competition between the elimination reaction and nucleophilic substitution reactions, ${{E}_{2}}\text{ and }{{S}_{N}}2$, and ${{E}_{1}}\text{ and }{{S}_{N}}1$reactions. Substitution reactions generally dominate and elimination occurs only during precise circumstances. Elimination reactions are generally favored over substitution reactions when
(i) steric hindrance around the $\alpha $-carbon increase.
(ii) a stronger base is used.
(iii) increase in temperature.
(iv) the base is a poor nucleophile.
Complete Step by step solution:
-When the reaction follows a one-step mechanism for elimination reaction, such reactions are said to be ${{E}_{2}}$reaction. When the reaction follows a two-step mechanism for elimination reaction, such reactions are said to be ${{E}_{1}}$reactions.
-The number 1 and 2 in ${{E}_{1}}$and ${{E}_{2}}$does not refers to the number of steps in the mechanisms rather refers to the kinetics of the reaction, that is ${{E}_{2}}$is a bimolecular (second-order) reaction while ${{E}_{1}}$is a unimolecular (first-order) reaction.
-${{E}_{1}}cb$ exists where the molecule can stabilize an anion but possesses a poor leaving group.
-Following the ${{E}_{1}}$mechanism, carbocation is formed from the heterolytic cleavage of the C-Br bond. This is a slow step, hence is the rate-determining step. The proton abstraction now occurs from the adjacent carbon resulting in the formation of an alkene. Thus, only halide affects the rate of reaction.
-Following ${{E}_{2}}$mechanism, both Br atom and Deuterium or Hydrogen atom leave simultaneously and thus affect the rate.
-Following ${{E}_{1}}cb$, the first step is the removal of proton resulting in carbanion formation followed by the cleavage of halide or leaving group. Since, ${{E}_{1}}cb$ is a two-step mechanism, an isotope effect will be present.
-Anti-elimination and ${{E}_{2}}$eliminations are the same.
Therefore the correct answer is option A.
Note: The reactivity of halogens, iodide, and bromine are being favoured for influencing the rate of reaction. Fluoride not being a good leaving group hence gives elimination reactions at slower rates than other halogens. There is a competition between the elimination reaction and nucleophilic substitution reactions, ${{E}_{2}}\text{ and }{{S}_{N}}2$, and ${{E}_{1}}\text{ and }{{S}_{N}}1$reactions. Substitution reactions generally dominate and elimination occurs only during precise circumstances. Elimination reactions are generally favored over substitution reactions when
(i) steric hindrance around the $\alpha $-carbon increase.
(ii) a stronger base is used.
(iii) increase in temperature.
(iv) the base is a poor nucleophile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




