
In the given reaction, ‘X’ will be:
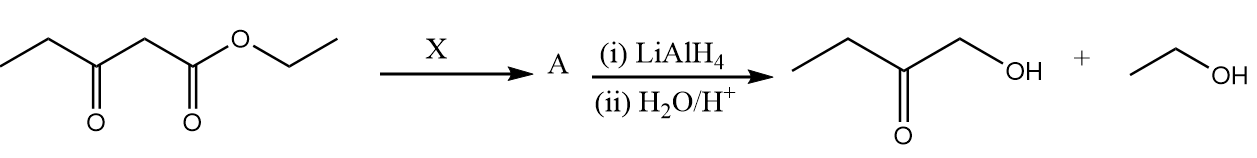
A. $ HCHO $
B.
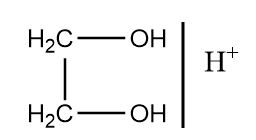
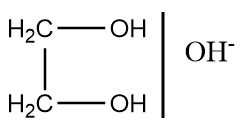
C.
D. $ HCN $
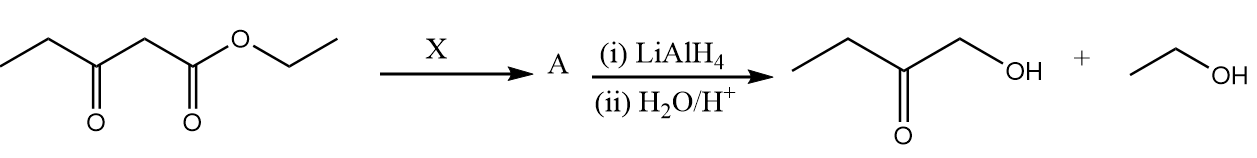


Answer
510.3k+ views
Hint: Lithium aluminium hydride is a strong, unselective reducing agent which is a common source for hydride ion and is commonly used in most of the organic reactions for reduction of aldehyde, esters, ketone, carboxylic acid, acid chloride and carboxylate salts to respective alcohols. Amides and nitriles can also be reduced to amines in the presence of lithium aluminium hydride.
Complete answer:
In the given reaction, the reactant consists of two functional groups which are ester and ketone group. But, after reaction it is clear that only the ester group is reduced to alcohol while ketone did not get reduced. This means the reagent ‘X’ used in the reaction was to protect the ketone group from getting reduced.
Therefore, among given options ethylene glycol in the presence of acidic medium is used as a reagent to protect the ketone group by forming ketal. The mechanism of reaction takes place as follows:
Step-1: Ethylene glycol attacks the given reactant in an acidic medium to form ketal along with removal of water. The reaction proceeds as follows:

Step-2: In the presence of lithium aluminium hydride, the ester group present in the compound converts into respective alcohols. The reaction takes place as follows:
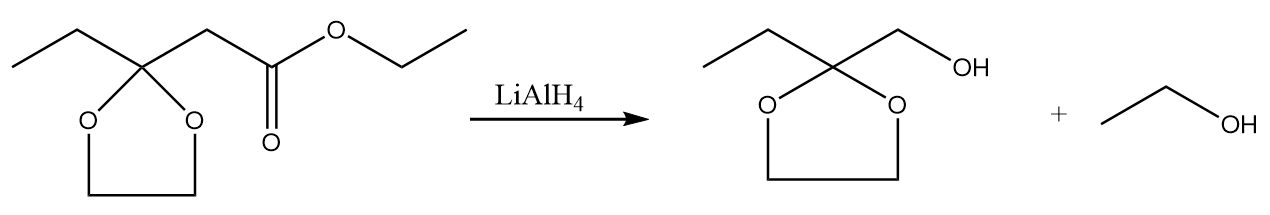
Step-3: When water reacts with the compound formed in the previous step, then the ketal group is converted to ketone along with the removal of ethylene glycol. The reaction takes place as follows:

Hence, the structure of ‘X’ used in the reaction is:
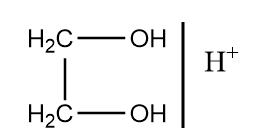
So, option (B) is the correct answer.
Note:
It is important to note that the acetal and ketal derived from aldehydes and ketones are base proof i.e., in the presence of base, no chemical change take place at this group and hence, these groups are widely used in the organic reactions to protect aldehyde and ketone group. Also remember that the formation of acetal and ketals does not take place in basic medium, therefore, option (c) is not applicable for given reaction conditions.
Complete answer:
In the given reaction, the reactant consists of two functional groups which are ester and ketone group. But, after reaction it is clear that only the ester group is reduced to alcohol while ketone did not get reduced. This means the reagent ‘X’ used in the reaction was to protect the ketone group from getting reduced.
Therefore, among given options ethylene glycol in the presence of acidic medium is used as a reagent to protect the ketone group by forming ketal. The mechanism of reaction takes place as follows:
Step-1: Ethylene glycol attacks the given reactant in an acidic medium to form ketal along with removal of water. The reaction proceeds as follows:

Step-2: In the presence of lithium aluminium hydride, the ester group present in the compound converts into respective alcohols. The reaction takes place as follows:

Step-3: When water reacts with the compound formed in the previous step, then the ketal group is converted to ketone along with the removal of ethylene glycol. The reaction takes place as follows:

Hence, the structure of ‘X’ used in the reaction is:

So, option (B) is the correct answer.
Note:
It is important to note that the acetal and ketal derived from aldehydes and ketones are base proof i.e., in the presence of base, no chemical change take place at this group and hence, these groups are widely used in the organic reactions to protect aldehyde and ketone group. Also remember that the formation of acetal and ketals does not take place in basic medium, therefore, option (c) is not applicable for given reaction conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




