
In the given reaction:
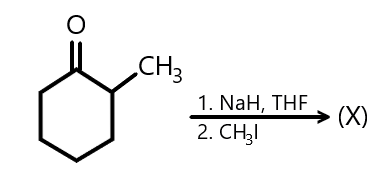
X will be:
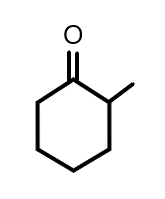
(A)
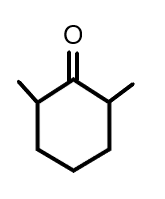
(B)
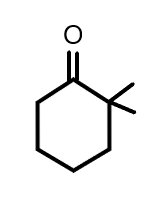
(C)
(D)






Answer
541.5k+ views
Hint :As we know that THF is tetrahydrofuran which is used as a solvent for resins such as photosensitive resins. It can be used as a solvent because at room temperature it has the ability to dissolve the resins but it is miscible with water and other organic solvents. It is used widely in Grignard and Wittig reactions as a solvent.
Complete Step By Step Answer:
As we know that Sodium hydride is the chemical compound which is used as a strong combustible base in organic synthesis. The sodium hydride is used in the reaction to deprotonate the compound so the hydrogen gas is released in the reaction. It then forms the nucleophilic alkoxide ion by performing the $ S{N_2} $ mechanism. Sodium hydride has high basicity but it is not a nucleophile. And along with Tetrahydrofuran, it forms an alkoxide ion. This ionic compound when reacts with methyl iodide results in the formation of di-methyl compound.
We also know that when the given reactant is reacted with $ NaH $ and $ THF $ , it results in the formation of the following compounds as shown below and on further reaction with methyl iodide, the final product is formed which is predicted as followed:

Therefore, we can say that the correct option for this answer is option ‘c’.
Note :
Always remember that sodium hydride is used as a desiccant or drying agent in the formation or manufacture of compounds in a laboratory. One of the important applications of sodium hydride is that it is used as a hydrogen storage agent in fuel cell vehicles. And Methyl iodide provides a methyl group to the compound.
Complete Step By Step Answer:
As we know that Sodium hydride is the chemical compound which is used as a strong combustible base in organic synthesis. The sodium hydride is used in the reaction to deprotonate the compound so the hydrogen gas is released in the reaction. It then forms the nucleophilic alkoxide ion by performing the $ S{N_2} $ mechanism. Sodium hydride has high basicity but it is not a nucleophile. And along with Tetrahydrofuran, it forms an alkoxide ion. This ionic compound when reacts with methyl iodide results in the formation of di-methyl compound.
We also know that when the given reactant is reacted with $ NaH $ and $ THF $ , it results in the formation of the following compounds as shown below and on further reaction with methyl iodide, the final product is formed which is predicted as followed:

Therefore, we can say that the correct option for this answer is option ‘c’.
Note :
Always remember that sodium hydride is used as a desiccant or drying agent in the formation or manufacture of compounds in a laboratory. One of the important applications of sodium hydride is that it is used as a hydrogen storage agent in fuel cell vehicles. And Methyl iodide provides a methyl group to the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




