
In the given reaction:
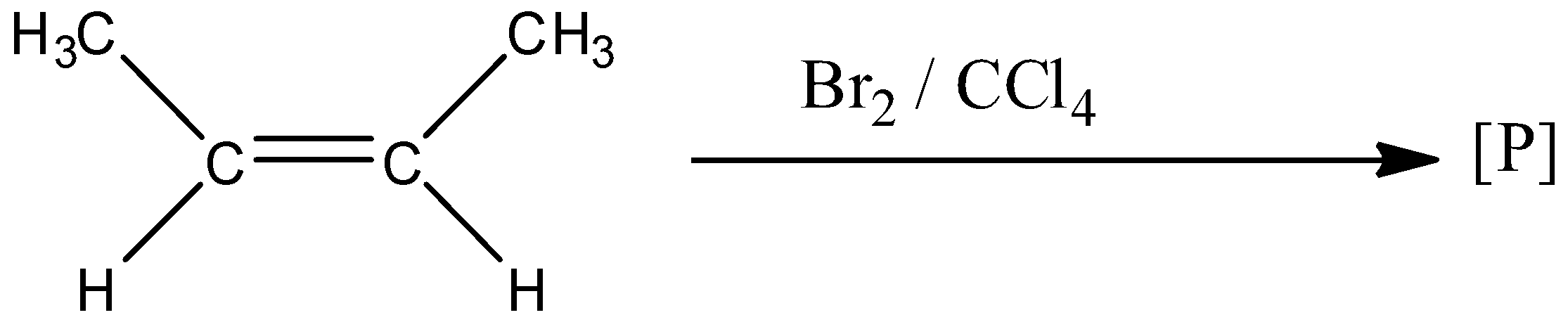
Product [P] will be:
(a)- Erythro-2, 3-dibromobutane
(b)- Threo-2, 3-dibromobutane
(c)- 1:1 mixture of Erythro-2, 3-dibromobutane and Threo-2, 3-dibromobutane
(d)- 2:1 mixture of Erythro-2, 3-dibromobutane and Threo-2, 3-dibromobutane
Answer
565.5k+ views
Hint: Erythro and threo are the configuration of the organic compound that tells the position of the substituents in the compound. If two same compounds are on the same side of the carbon atoms then it is erythro configuration and if two same compounds are on the opposite side of the carbon atoms then it is threo configuration.
Complete s answer:
The given compound in the question is the cis form of but-2-ene. And this compound is treated with bromine in the presence of carbon tetrachloride. So two bromine attaches to the double bond leading to an additional reaction. But both bromines don't attach on the same side of the side. Or we can say that one bromine attaches above the plane of the double bond and another bromine attaches below the plane of the double bond. The reaction is given below:
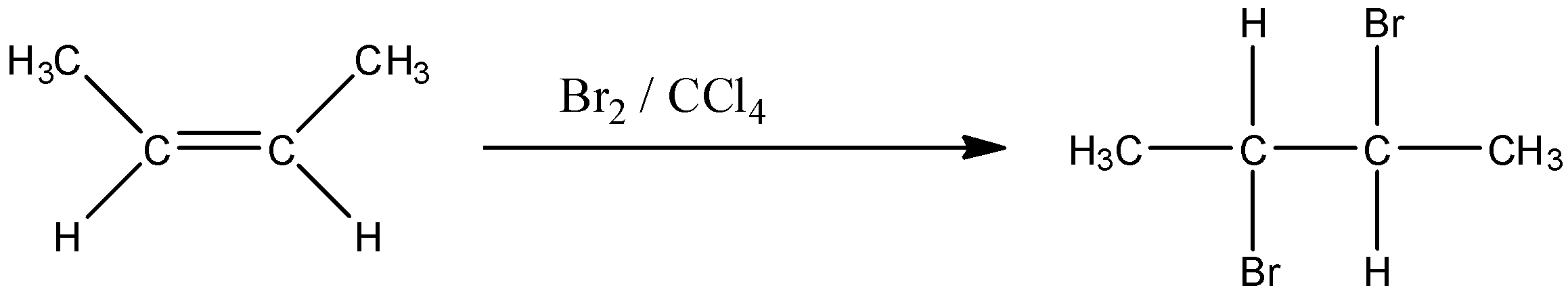
So in this compound, both the bromine atoms are in the opposite direction.
Erythro and threo are the configuration of the organic compound that tells the position of the substituents in the compound. If two same compounds are on the same side of the carbon atoms then it is erythro configuration and if two same compounds are on the opposite side of the carbon atoms then it is threo configuration.
So the above compound is Threo-2, 3-dibromobutane.
Therefore, the correct answer is option (b)- Threo-2, 3-dibromobutane.
Note:
Erythro and Threo configuration is mostly in the naming of carbohydrates, for defining the hydroxyl group on either side of the carbon atoms. In this type of addition, reaction fluorine doesn't react and iodine is the most reactive.
Complete s answer:
The given compound in the question is the cis form of but-2-ene. And this compound is treated with bromine in the presence of carbon tetrachloride. So two bromine attaches to the double bond leading to an additional reaction. But both bromines don't attach on the same side of the side. Or we can say that one bromine attaches above the plane of the double bond and another bromine attaches below the plane of the double bond. The reaction is given below:
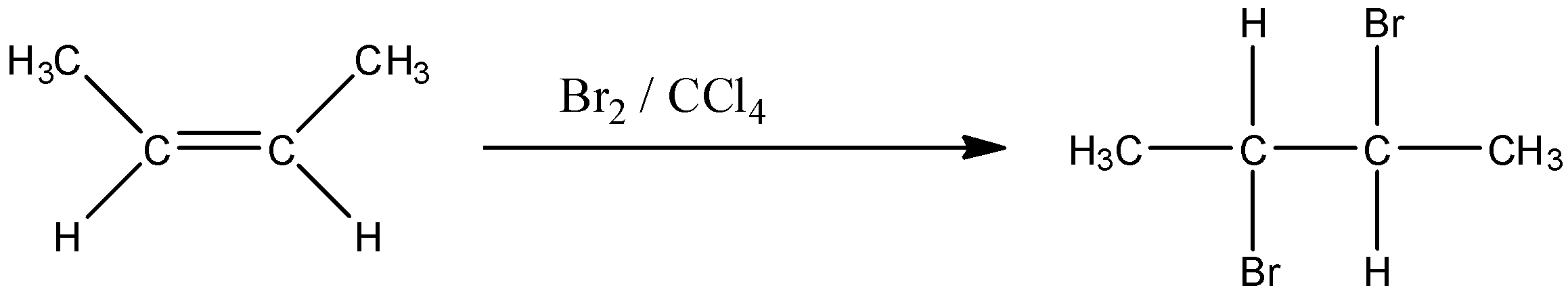
So in this compound, both the bromine atoms are in the opposite direction.
Erythro and threo are the configuration of the organic compound that tells the position of the substituents in the compound. If two same compounds are on the same side of the carbon atoms then it is erythro configuration and if two same compounds are on the opposite side of the carbon atoms then it is threo configuration.
So the above compound is Threo-2, 3-dibromobutane.
Therefore, the correct answer is option (b)- Threo-2, 3-dibromobutane.
Note:
Erythro and Threo configuration is mostly in the naming of carbohydrates, for defining the hydroxyl group on either side of the carbon atoms. In this type of addition, reaction fluorine doesn't react and iodine is the most reactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




