
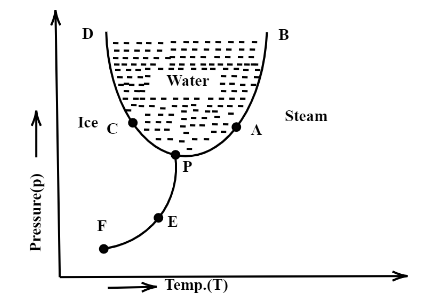
In the given pressure- temperature diagram, for water, which point indicates triple point?
(A) A
(B) C
(C) P
(D) E

Answer
478.2k+ views
Hint: The triple point of a substance is the temperature and pressure at which its three phases (gas, liquid, and solid) coexist in thermodynamic equilibrium, according to thermodynamics. The temperature and pressure at which the sublimation, fusion, and vaporisation curves all intersect. This point is unique for different substances.
Complete Step By Step Answer:
The triple point is the point at which all three phases of matter coexist. It is easy to convert the matter into another phase at this point by making a small change to the system. In the diagram given above the point at which all the three phases of water, that is, vapour, liquid and solid, meet each other is at the point P. Hence P is the triple point.
Every substance has a unique triple point. The triple point of water is important because it is used as a standard reference point for defining the Kelvin temperature scale. Because the triple point is a point, the three phases can only exist at one temperature and pressure. This information is frequently useful in identifying compounds or solving problems.
The correct answer is option C, P is the triple point of water.
Note:
For substances having many polymorphs, a triple point may involve more than one solid phase in addition to the solid, liquid, and gas phases. Helium-4 is a unique instance since it has a triple point comprising two fluid phases.
Complete Step By Step Answer:
The triple point is the point at which all three phases of matter coexist. It is easy to convert the matter into another phase at this point by making a small change to the system. In the diagram given above the point at which all the three phases of water, that is, vapour, liquid and solid, meet each other is at the point P. Hence P is the triple point.
Every substance has a unique triple point. The triple point of water is important because it is used as a standard reference point for defining the Kelvin temperature scale. Because the triple point is a point, the three phases can only exist at one temperature and pressure. This information is frequently useful in identifying compounds or solving problems.
The correct answer is option C, P is the triple point of water.
Note:
For substances having many polymorphs, a triple point may involve more than one solid phase in addition to the solid, liquid, and gas phases. Helium-4 is a unique instance since it has a triple point comprising two fluid phases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




