
In the given molecule, which atom/group is below the plane of the paper?
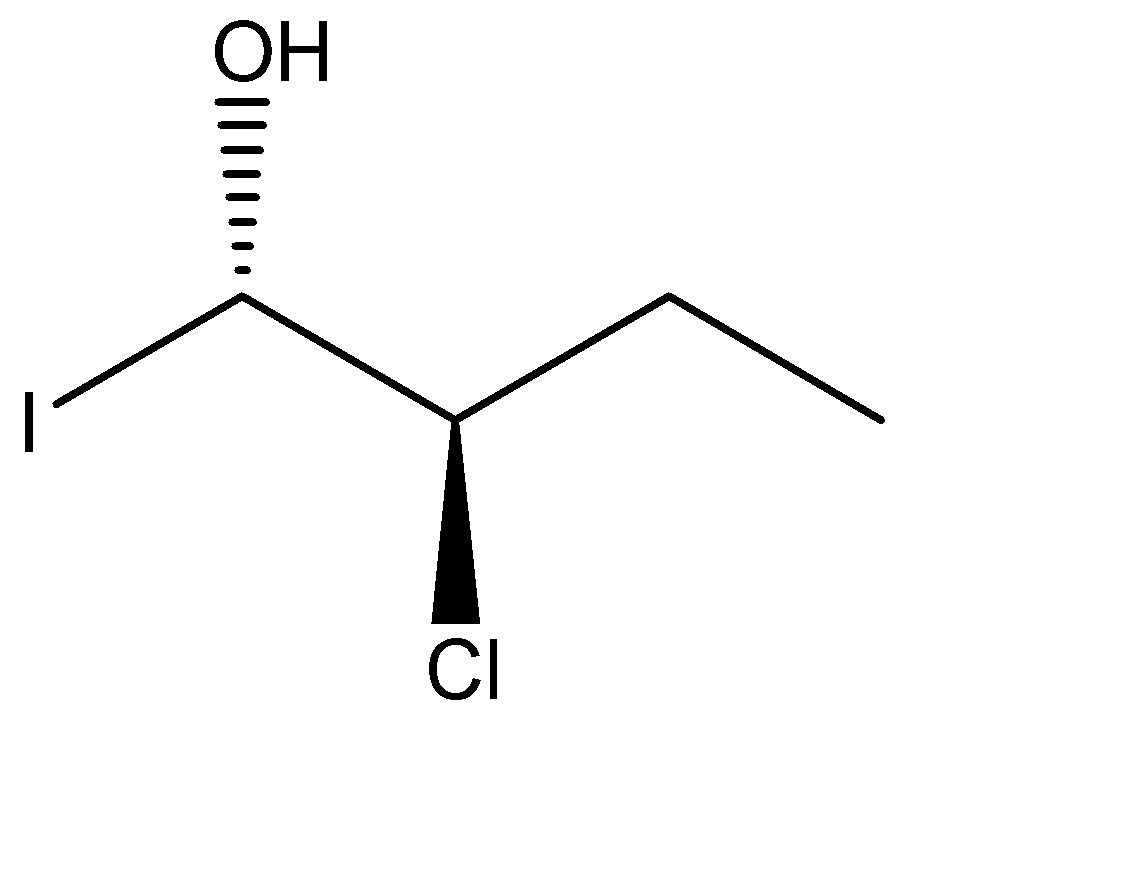
(A) Chlorine
(B) Iodine
(C) Hydroxyl
(D) Ethyl

Answer
530.4k+ views
Hint: As we know that representation of molecules in various projections helps us a lot while solving the question especially in organic chemistry. The molecule given in the question is drawn in wedge and dash projection. So to solve this question, let us understand about the wedge and dash projection.
Complete answer:
Let us begin with the concept of wedge and dash projection:-
As we know that wedge and dash projections are used to represent three-dimensional structures of compounds on a two dimensional paper in organic chemistry. There are majorly three types of bonds in the wedge and dash projections which are shown below:-
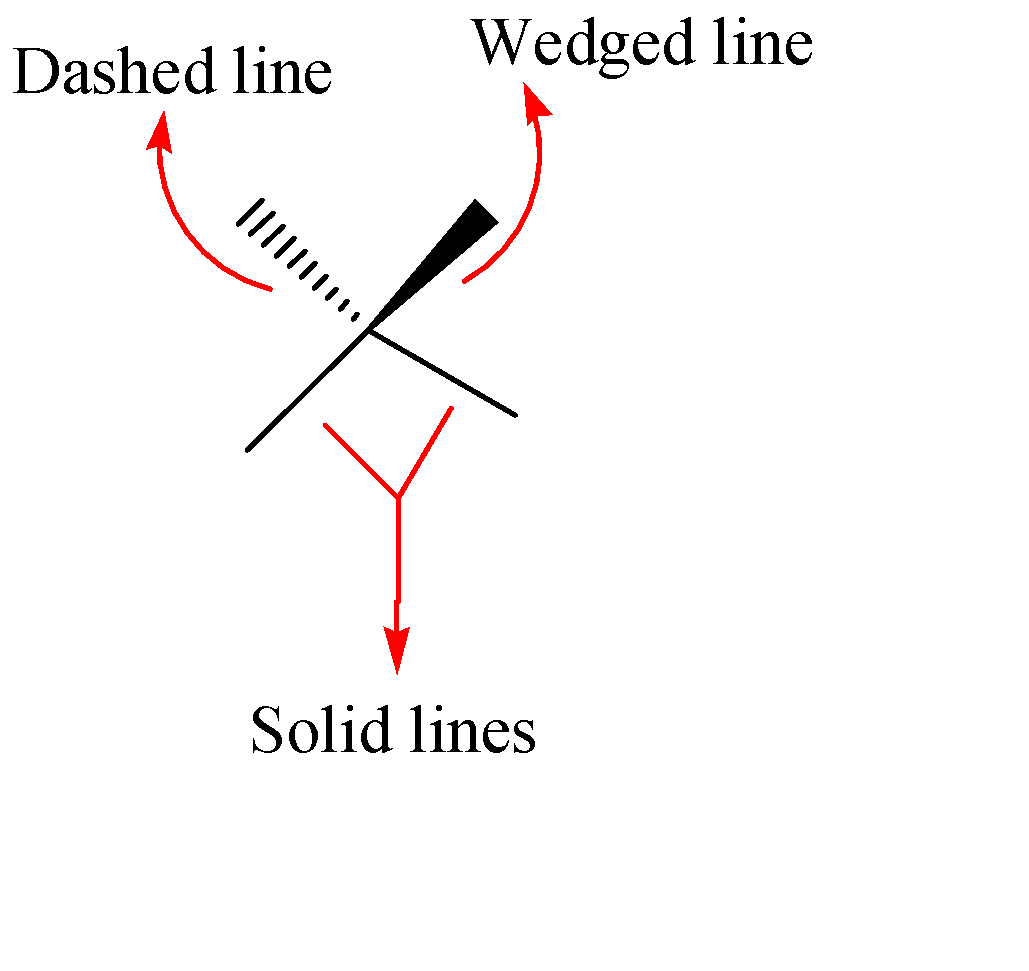
Solid Lines: These lines represent the atoms or groups connected in the plane of the paper.
Wedges: These are wedge shaped bonds which shows the connected atoms or groups coming out of the plane i.e., towards the viewer.
Dashes: Dash bond represents the connected atoms or groups behind the paper i.e., away from the viewer.
-Now we will apply the above explanation to the molecule given in the question:-
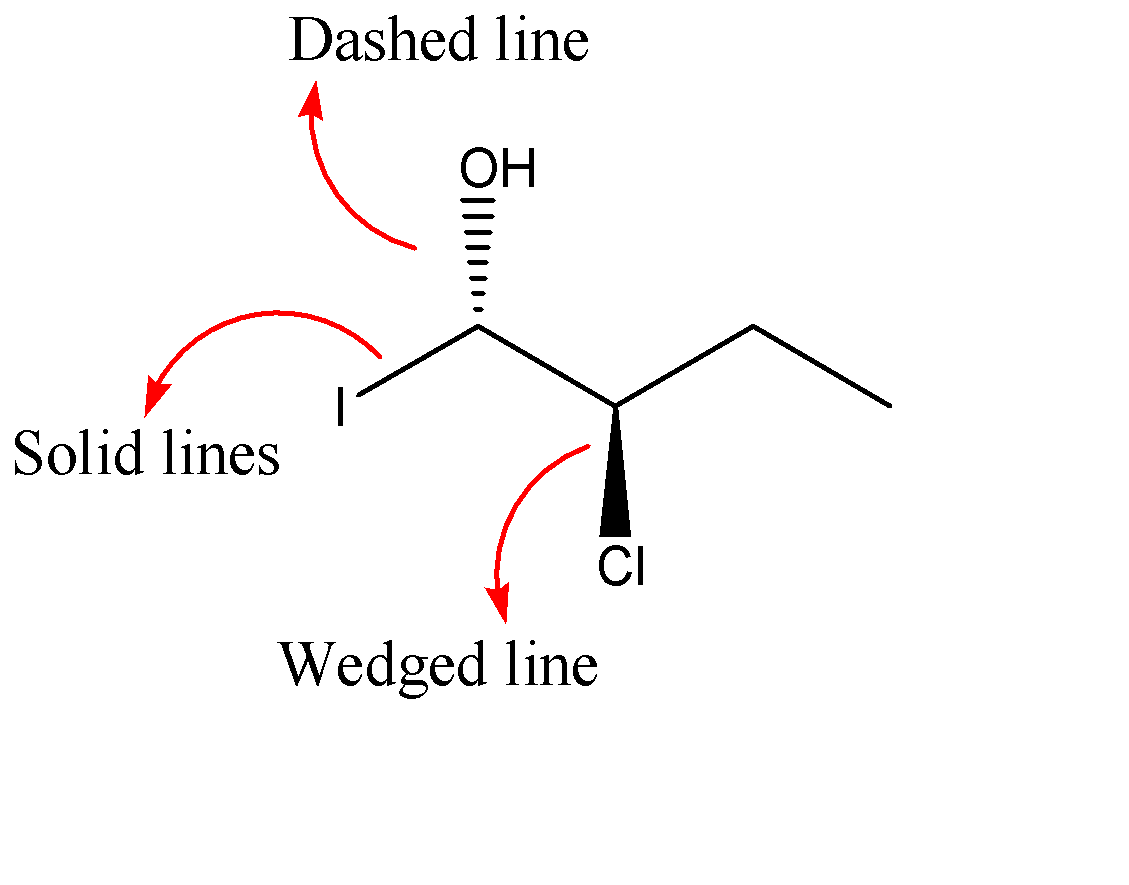
As we can see that the chlorine group is above the plane of paper and the hydroxyl group is below the plane of the paper. But the Iodine group is represented with the help of a solid line which means it is on the plane of the paper.
Hence, the atom/group which is below the plane of the paper is: (C) hydroxyl
Note:
-For solving such types of questions, we must have a proper understanding of all the projections by which a molecule can be represented because they are also helpful in the organic reactions.
-Majorly we prefer to draw wedge and dash projection to represent a molecule in three dimensions, but the other projections are Newman projection, sawhorse diagram whereas Fischer projection is used to represent a molecule in two dimensions.
Complete answer:
Let us begin with the concept of wedge and dash projection:-
As we know that wedge and dash projections are used to represent three-dimensional structures of compounds on a two dimensional paper in organic chemistry. There are majorly three types of bonds in the wedge and dash projections which are shown below:-

Solid Lines: These lines represent the atoms or groups connected in the plane of the paper.
Wedges: These are wedge shaped bonds which shows the connected atoms or groups coming out of the plane i.e., towards the viewer.
Dashes: Dash bond represents the connected atoms or groups behind the paper i.e., away from the viewer.
-Now we will apply the above explanation to the molecule given in the question:-

As we can see that the chlorine group is above the plane of paper and the hydroxyl group is below the plane of the paper. But the Iodine group is represented with the help of a solid line which means it is on the plane of the paper.
Hence, the atom/group which is below the plane of the paper is: (C) hydroxyl
Note:
-For solving such types of questions, we must have a proper understanding of all the projections by which a molecule can be represented because they are also helpful in the organic reactions.
-Majorly we prefer to draw wedge and dash projection to represent a molecule in three dimensions, but the other projections are Newman projection, sawhorse diagram whereas Fischer projection is used to represent a molecule in two dimensions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




