
In the formation of oxygen molecule
a) Electronic configuration of nearest inert gas attained.
b) How many electrons are shared/ transferred in bond formation?
c) Which type of bonds these compounds form?
d) Draw the orbital diagrams?
Answer
512.7k+ views
Hint: Oxygen exists in the form of a diatomic oxygen molecule. The electronic configuration consists of filling of electrons according to the Aufbau principle. A bond is formed by both sharing and transfer of electrons, called covalent and ionic bonds respectively. The orbital diagram of any molecule shows the formation of the molecule by mixing of orbitals.
Complete answer:
a) Inert gases have fully filled electronic configuration. Oxygen has a configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$ with atomic number 8. Its nearest inert gas is neon with atomic number 10 that has configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}$.
b) Oxygen molecules are formed by the two oxygen atoms. In order to form a chemical bond, the atoms need to attain fully filled electronic configuration. The oxygen atom requires 2 electrons to acquire an inert configuration; hence 2 electrons are shared between them in bond formation.
c) When electrons in an atom are shared to form chemical compounds, then the bonds are called covalent bonds.
d) The orbital diagram of${{O}_{2}}$ molecule is as follows:
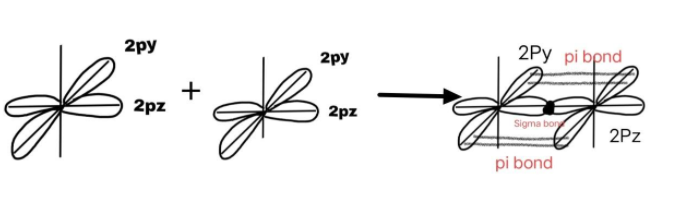
The 2 oxygen atoms combine and form one $\pi $(pi) due to overlapping of p – orbitals and one$\sigma $ (sigma) bond which is the base of pi – bond, so the molecule has a double bond as $O=O$.
Note:
The oxygen molecule has covalent bonds as they have the same electronegativity so they have sharing of electrons and not transfer of electrons. The formation of a molecule consists of mixing of outer orbitals, so p – orbitals are shown in the orbital diagram to form a bond in the oxygen molecule.
Complete answer:
a) Inert gases have fully filled electronic configuration. Oxygen has a configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$ with atomic number 8. Its nearest inert gas is neon with atomic number 10 that has configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}$.
b) Oxygen molecules are formed by the two oxygen atoms. In order to form a chemical bond, the atoms need to attain fully filled electronic configuration. The oxygen atom requires 2 electrons to acquire an inert configuration; hence 2 electrons are shared between them in bond formation.
c) When electrons in an atom are shared to form chemical compounds, then the bonds are called covalent bonds.
d) The orbital diagram of${{O}_{2}}$ molecule is as follows:

The 2 oxygen atoms combine and form one $\pi $(pi) due to overlapping of p – orbitals and one$\sigma $ (sigma) bond which is the base of pi – bond, so the molecule has a double bond as $O=O$.
Note:
The oxygen molecule has covalent bonds as they have the same electronegativity so they have sharing of electrons and not transfer of electrons. The formation of a molecule consists of mixing of outer orbitals, so p – orbitals are shown in the orbital diagram to form a bond in the oxygen molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




