
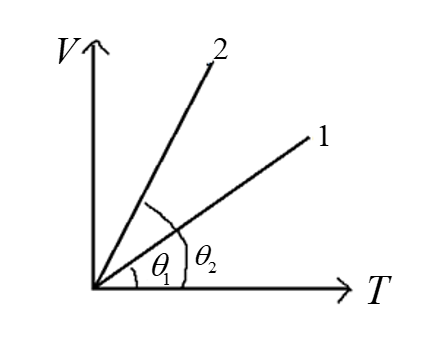
In the following V-T diagram, what is the relation between ${P_1}$ and ${P_2}$.
A) ${P_1} = {P_2}$
B) ${P_2} > {P_1}$
C) ${P_2} < {P_1}$
D) Cannot be predicted.

Answer
580.2k+ views
Hint: A gas which obeys Boyle’s law and Charles’s law strictly at all temperature and pressure is called a perfect or an ideal gas. When heat is supplied to gas at constant pressure, it is used to increase the temperature of the gas and in doing work due to expansion at constant pressure.
Complete step by step answer:
According to Charles’s law at constant pressure, it states that the volume of a given mass of a gas is directly proportional to its temperature.
That is,$V \propto T$ at constant pressure.
$ \Rightarrow \dfrac{V}{T} = {\text{constant}}$ ……………..(1)
Boyle’s law states that the volume of a given mass of a gas is inversely proportional to its pressure $P$ at a constant temperature.
$V \propto \dfrac{1}{P}$ At, constant T.
$ \Rightarrow PV = {\text{constant}}$ ………………….(2)
Combining equation (1) and (2) we get,
$\dfrac{{PV}}{T} = {\text{constant}}$
$\dfrac{{PV}}{T} = R$
For $n$ number of moles, we can write this as,
$PV = nRT$
Where, $R$ is the universal gas constant and $n$ is the number of moles in the sample of a gas.
From the given figure, we have a \[V - T\] diagram for two different pressures.
Slope of the graph is given by,
Slope$ = \dfrac{{y - axis}}{{x - axis}}$
$slope = \dfrac{{\Delta V}}{{\Delta T}}$ ………………..(3)
Now consider ideal gas equation, $PV = nRT$
Rearranging the above equation, we get
$\dfrac{V}{T} = \dfrac{{nR}}{P}$
From equation (3) we can write it as,
$slope = \dfrac{{nR}}{P}$
Here $n$ and $R$ are constants.
Therefore, $slope \propto \dfrac{1}{P}$
The slope of the V-T graph is inversely proportional to the pressure.
For $P_1$, ${(slope)_1} \propto \dfrac{1}{{{P_1}}}$
Here, the slope is less thus the value of ${P_1}$ is more.
Similarly, for ${P_2}$, ${(slope)_2} \propto \dfrac{1}{{{P_2}}}$
Here, the slope is more thus, ${P_2}$ is less.
Now analyze the result we get, ${P_2}$ is less than ${P_1}$.
$ \Rightarrow {P_2} < {P_1}$
$\therefore $ The correct option is (C).
Note:
Isobaric process: The process in which a system undergoes a change in volume and temperature at constant pressure by the exchange of heat energy with the surroundings is called the isobaric process.
Isochoric process: The process in which a system undergoes a change in pressure and temperature at constant volume by the exchange of heat energy with the surrounding is called the isochoric process.
The universal gas constant is the constant for one mole of a gas. It is the same for all gases, since, at the same temperature and pressure, one mole of any gas occupies the same volume.
For an ideal gas internal energy of a gas depends only on temperature.
Complete step by step answer:
According to Charles’s law at constant pressure, it states that the volume of a given mass of a gas is directly proportional to its temperature.
That is,$V \propto T$ at constant pressure.
$ \Rightarrow \dfrac{V}{T} = {\text{constant}}$ ……………..(1)
Boyle’s law states that the volume of a given mass of a gas is inversely proportional to its pressure $P$ at a constant temperature.
$V \propto \dfrac{1}{P}$ At, constant T.
$ \Rightarrow PV = {\text{constant}}$ ………………….(2)
Combining equation (1) and (2) we get,
$\dfrac{{PV}}{T} = {\text{constant}}$
$\dfrac{{PV}}{T} = R$
For $n$ number of moles, we can write this as,
$PV = nRT$
Where, $R$ is the universal gas constant and $n$ is the number of moles in the sample of a gas.
From the given figure, we have a \[V - T\] diagram for two different pressures.
Slope of the graph is given by,
Slope$ = \dfrac{{y - axis}}{{x - axis}}$
$slope = \dfrac{{\Delta V}}{{\Delta T}}$ ………………..(3)
Now consider ideal gas equation, $PV = nRT$
Rearranging the above equation, we get
$\dfrac{V}{T} = \dfrac{{nR}}{P}$
From equation (3) we can write it as,
$slope = \dfrac{{nR}}{P}$
Here $n$ and $R$ are constants.
Therefore, $slope \propto \dfrac{1}{P}$
The slope of the V-T graph is inversely proportional to the pressure.
For $P_1$, ${(slope)_1} \propto \dfrac{1}{{{P_1}}}$
Here, the slope is less thus the value of ${P_1}$ is more.
Similarly, for ${P_2}$, ${(slope)_2} \propto \dfrac{1}{{{P_2}}}$
Here, the slope is more thus, ${P_2}$ is less.
Now analyze the result we get, ${P_2}$ is less than ${P_1}$.
$ \Rightarrow {P_2} < {P_1}$
$\therefore $ The correct option is (C).
Note:
Isobaric process: The process in which a system undergoes a change in volume and temperature at constant pressure by the exchange of heat energy with the surroundings is called the isobaric process.
Isochoric process: The process in which a system undergoes a change in pressure and temperature at constant volume by the exchange of heat energy with the surrounding is called the isochoric process.
The universal gas constant is the constant for one mole of a gas. It is the same for all gases, since, at the same temperature and pressure, one mole of any gas occupies the same volume.
For an ideal gas internal energy of a gas depends only on temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




