
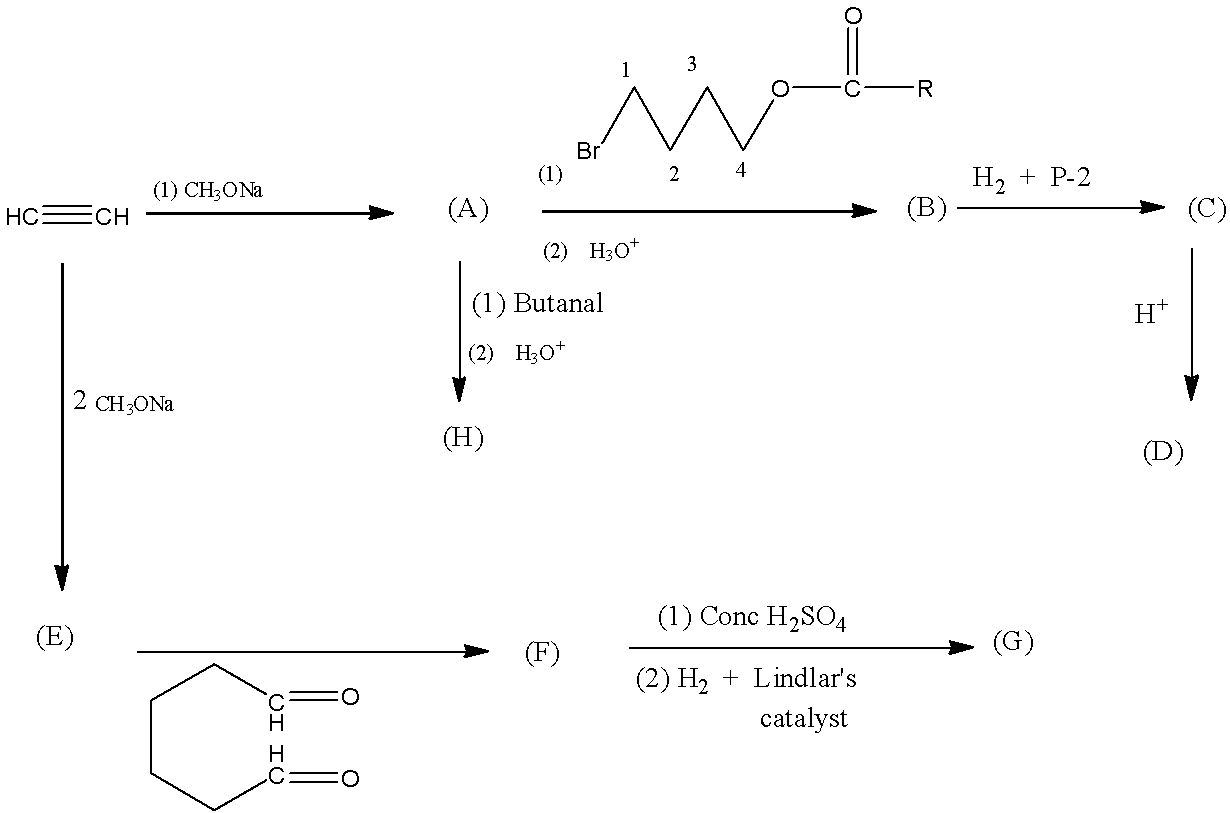
In the following sequence of reactions, products (A) to (H) are formed. Then the compound (G) is:


Answer
590.7k+ views
Hint: Hydrogen and Lindlar’s catalyst is used to reduce the triple bonds to double bond, so the compound will not have any triple bond. Concentrated sulfuric acid is used to remove water from the molecule.
Complete step by step answer:
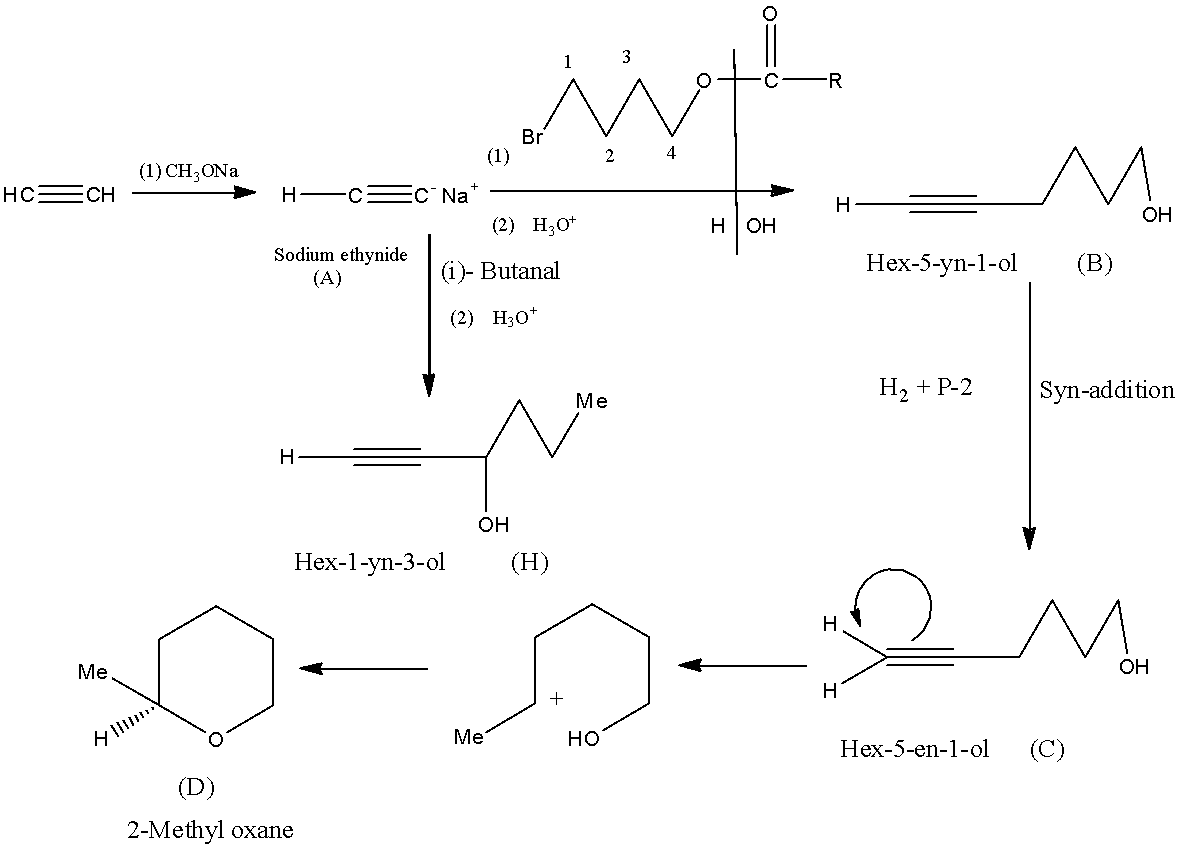
When ethyne is reacted with 1 mole of sodium methoxide and sodium ethoxide is formed by the removal of one hydrogen from the ethyne.
When (A) is converted into (B), the first catalyst gets converted into alcohol with the help of the second catalyst and gets attached at the place of sodium, hence forming Hex-5-yn-1-ol.
Product (C), Hex-5-en-1-ol is formed from (B) by the Syn-addition. Now (C) is converted into (D) by the action of acid which forms a cyclic structure called 2-Methyl oxane.
Compound (A) is converted into (H), the first catalyst gets converted into alcohol with the help of the second catalyst and gets attached at the place of sodium, hence forming Hex-1-yn-3-ol.
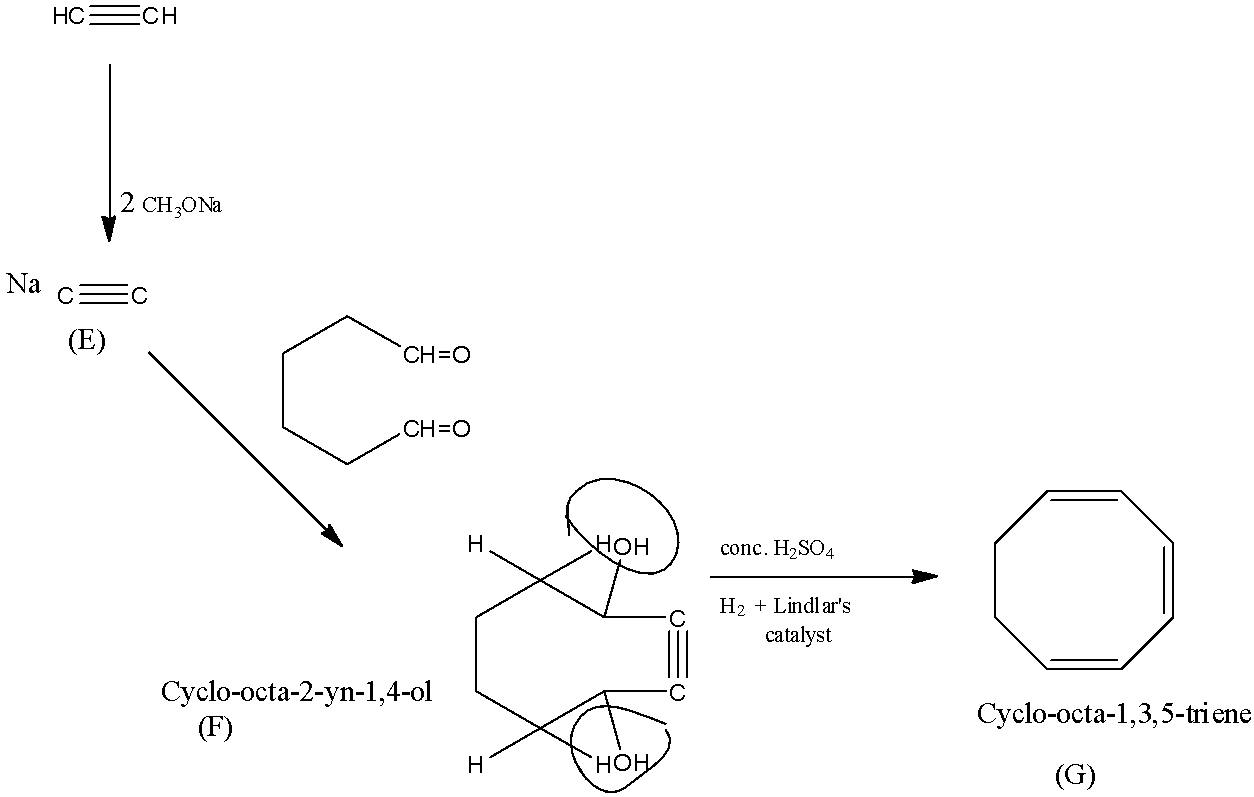
When 2 moles of sodium methoxide are reacted with ethyne, both the hydrogen in ethyne is replaced with sodium atom which is the (E).
The compound (E) is converted into (F), forming cyclo-octa-2-yn-1,4-diol.
Now with concentrated sulfuric acid 2 moles of water is removed and with hydrogen and Lindlar's catalyst the triple bond is reduced to the double bond
So the reactions are:
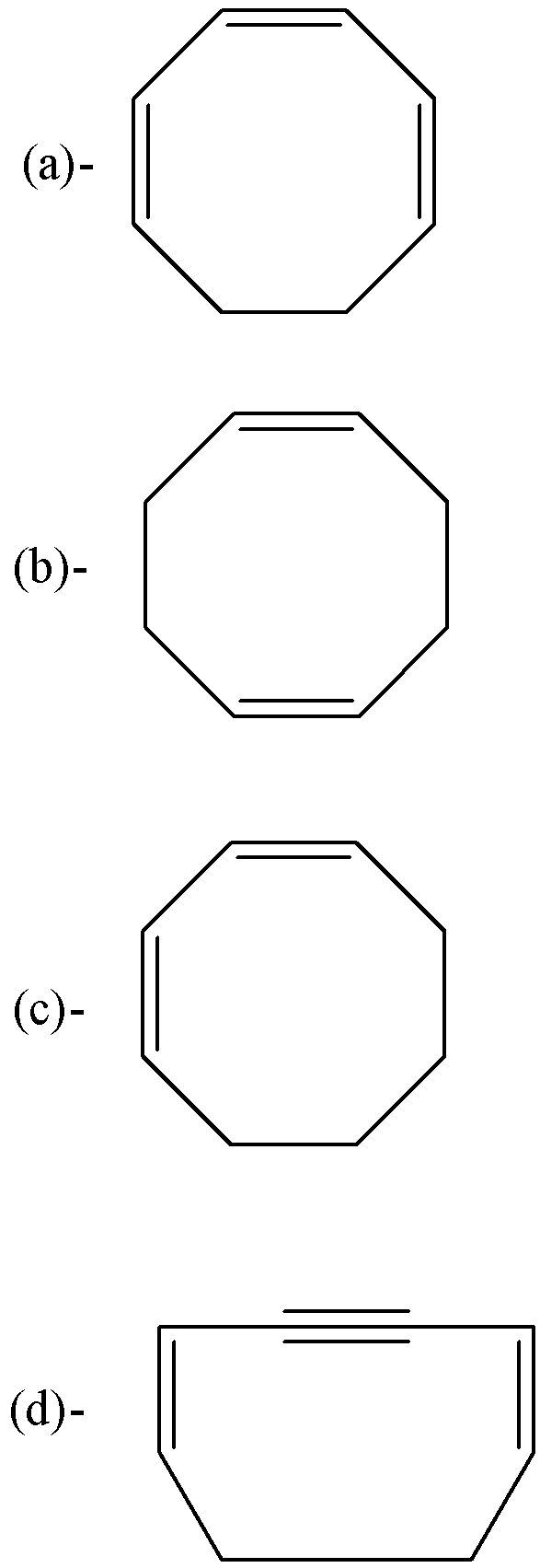
So, the correct answer is “Option A”.
Note: When the alkynes are reacted with Lindlar’s catalyst the alkene formed is cis-alkene but if the alkynes are reduced with sodium in liquid ammonia (Birch reduction), trans-alkene is the major product.
Complete step by step answer:
When ethyne is reacted with 1 mole of sodium methoxide and sodium ethoxide is formed by the removal of one hydrogen from the ethyne.
When (A) is converted into (B), the first catalyst gets converted into alcohol with the help of the second catalyst and gets attached at the place of sodium, hence forming Hex-5-yn-1-ol.
Product (C), Hex-5-en-1-ol is formed from (B) by the Syn-addition. Now (C) is converted into (D) by the action of acid which forms a cyclic structure called 2-Methyl oxane.
Compound (A) is converted into (H), the first catalyst gets converted into alcohol with the help of the second catalyst and gets attached at the place of sodium, hence forming Hex-1-yn-3-ol.
When 2 moles of sodium methoxide are reacted with ethyne, both the hydrogen in ethyne is replaced with sodium atom which is the (E).
The compound (E) is converted into (F), forming cyclo-octa-2-yn-1,4-diol.
Now with concentrated sulfuric acid 2 moles of water is removed and with hydrogen and Lindlar's catalyst the triple bond is reduced to the double bond
So the reactions are:


So, the correct answer is “Option A”.
Note: When the alkynes are reacted with Lindlar’s catalyst the alkene formed is cis-alkene but if the alkynes are reduced with sodium in liquid ammonia (Birch reduction), trans-alkene is the major product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




