
In the following reaction which one of the following options is correct?
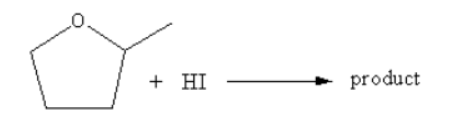
A)
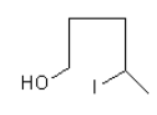
B)
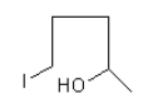
C)
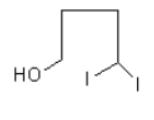
D)
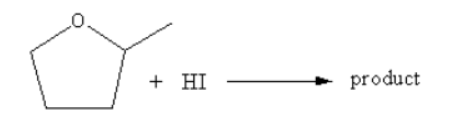




Answer
571.8k+ views
Hint: Tetrahydrofuran gives ring opening reactions in presence of hydrogen halide. The oxygen atom of tetrahydrofuran has a lone pair, so it is reactive for protonation. The halide prefers the attacks from the less hindered side of the ring.
Complete step by step solution:
Tetrahydro $ - 2 - $ methylfuran gives electrophilic addition reactions with hydrogen iodide.
In the overall reaction, the oxygen-carbon bond breaks and hydrogen and iodine get attached.
In the first step, oxygen atoms of tetrahydro $ - 2 - $ methylfuran have lone pairs, so they attack on hydrogen and abstract a proton from hydrogen iodide and get protonated. A negatively charged iodide ion also forms.
The ion having negative charge works as a nucleophile.
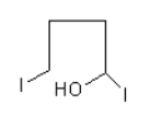
Then nucleophile iodide ion from less hindered site attacks on the nearby carbon of the –OH group, so ring opening takes place and the product $5 - $ idopentan $ - 2 - $ol forms.
As only one molecule of hydrogen iodide is adding so, the option (C) and (D) are incorrect.
The direction of attack and ring opening is important because it decides the stereo of the product. Product (a) and (b) are the same but the iodide ion attacks from outside, so the iodide group will remain outside and hydroxyl –OH will remain inside.
Therefore, option (B) is correct.
Note: Electrophilic addition takes place first, so this is an electrophilic addition reaction. This is a ring opening reaction. In ring opening reaction, nucleophile attacks from the less hindered side of the ring. The reaction is stereochemistry remains maintained. Tetrahydro $ - 2 - $ methylfuran works as Lewis base in organometallic reaction. It also works as a solvent in laboratory applications.
Complete step by step solution:
Tetrahydro $ - 2 - $ methylfuran gives electrophilic addition reactions with hydrogen iodide.
In the overall reaction, the oxygen-carbon bond breaks and hydrogen and iodine get attached.
In the first step, oxygen atoms of tetrahydro $ - 2 - $ methylfuran have lone pairs, so they attack on hydrogen and abstract a proton from hydrogen iodide and get protonated. A negatively charged iodide ion also forms.
The ion having negative charge works as a nucleophile.
Then nucleophile iodide ion from less hindered site attacks on the nearby carbon of the –OH group, so ring opening takes place and the product $5 - $ idopentan $ - 2 - $ol forms.
As only one molecule of hydrogen iodide is adding so, the option (C) and (D) are incorrect.
The direction of attack and ring opening is important because it decides the stereo of the product. Product (a) and (b) are the same but the iodide ion attacks from outside, so the iodide group will remain outside and hydroxyl –OH will remain inside.
Therefore, option (B) is correct.
Note: Electrophilic addition takes place first, so this is an electrophilic addition reaction. This is a ring opening reaction. In ring opening reaction, nucleophile attacks from the less hindered side of the ring. The reaction is stereochemistry remains maintained. Tetrahydro $ - 2 - $ methylfuran works as Lewis base in organometallic reaction. It also works as a solvent in laboratory applications.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




