
In the following reaction which of the following products can not form?
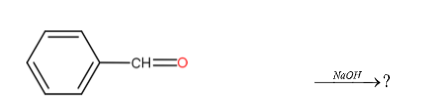
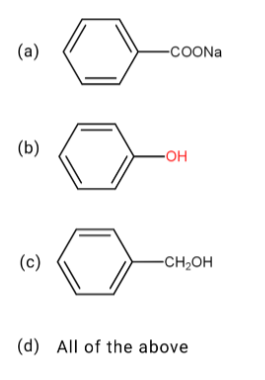
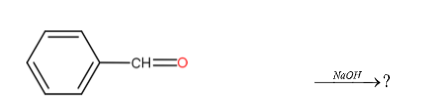

Answer
570k+ views
Hint: When we use condensed aldehydes or ketones in presence of sodium hydroxide there can only be two reactions that take place: aldol condensation or Cannizzaro reaction, depending upon the factors we should choose one.
Complete Step by step answer: So first of all we should identify which condensation reaction takes place here. Let us look into its $\alpha $-hydrogen of benzaldehyde, we can see the $\alpha $-carbon atom is attached to its neighbouring benzene carbons hence there is no space to accommodate the $\alpha $-hydrogen atom. So we can say that there is no $\alpha $-hydrogen atom in benzaldehyde. Therefore, the reaction will be Cannizzaro reaction.
It is a self oxidation-reduction reaction in which aldehydes that do not have any $\alpha $-hydrogen atom undergo disproportionation reaction (i.e., self-redox reaction) in the presence of 50% aqueous or ethanolic solution of alkali in which one of the molecules being reduced to alcohol and other being oxidised to the salt of the corresponding acid. So when benzaldehyde is reacted with NaOH, we get two substance one of them will its alcohol compound and the other one is its salt of corresponding acid.
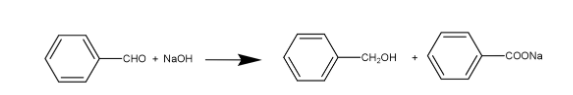
We can see that when benzaldehyde is treated with NaOH we get the two compounds. One is the reduced one to its corresponding alcohol i.e., benzyl alcohol and the other one is its oxidised form i.e., sodium benzoate.
It is clearly evident that phenol is not obtained from the above reaction.
Therefore, option (b) is correct.
Note: We cannot produce phenol from Cannizzaro reaction since the reaction happens at the functional group it cannot attack the benzene ring, that is why the phenol is not obtained from this reaction. It may come to our mind that the hydroxyl group may attach to the benzene ring, but it does not happen here.
Complete Step by step answer: So first of all we should identify which condensation reaction takes place here. Let us look into its $\alpha $-hydrogen of benzaldehyde, we can see the $\alpha $-carbon atom is attached to its neighbouring benzene carbons hence there is no space to accommodate the $\alpha $-hydrogen atom. So we can say that there is no $\alpha $-hydrogen atom in benzaldehyde. Therefore, the reaction will be Cannizzaro reaction.
It is a self oxidation-reduction reaction in which aldehydes that do not have any $\alpha $-hydrogen atom undergo disproportionation reaction (i.e., self-redox reaction) in the presence of 50% aqueous or ethanolic solution of alkali in which one of the molecules being reduced to alcohol and other being oxidised to the salt of the corresponding acid. So when benzaldehyde is reacted with NaOH, we get two substance one of them will its alcohol compound and the other one is its salt of corresponding acid.

We can see that when benzaldehyde is treated with NaOH we get the two compounds. One is the reduced one to its corresponding alcohol i.e., benzyl alcohol and the other one is its oxidised form i.e., sodium benzoate.
It is clearly evident that phenol is not obtained from the above reaction.
Therefore, option (b) is correct.
Note: We cannot produce phenol from Cannizzaro reaction since the reaction happens at the functional group it cannot attack the benzene ring, that is why the phenol is not obtained from this reaction. It may come to our mind that the hydroxyl group may attach to the benzene ring, but it does not happen here.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




