
In the following reaction, ether linkage is cleaved by $PC{l_5}$
$R - O - R' + PC{l_5} \to RCl + R'Cl + POC{l_3}$
If ${C_5}{H_{12}}O$ (ether) forms 2-chloropropane as one of the products, then ether
A) 1-ethoxypropane
B) 2-ethoxypropane
C) 1-methoxybutane
D) 2-methoxypropane
Answer
582.9k+ views
Hint: Given the molecular formula of the ether in the question is ${C_5}{H_{12}}O$ and when this ether is cleaved by$PC{l_5}$, we get 2-chloropropane as one of the products. You must know the chemical formula of 2-chloropropane and it is $C{H_3} - CH(Cl) - C{H_3}$. Do the chemical reaction of ${C_5}{H_{12}}O$ with $PC{l_5}$ and examine the products.
Complete step by step solution:
In the question, it is given that ether linkage ($R-O-R’$) is cleaved by $PC{l_5}$ as follows:
$R - O - R' + PC{l_5} \to RCl + R'Cl + POC{l_3}$
Now, the molecular formula of the given ether in the question is ${C_5}{H_{12}}O$
When ${C_5}{H_{12}}O$ is cleaved by $PC{l_5}$, we get 2-chloropropane as one of the products.
As you know, the molecular formula of 2-chloropropane is: $C{H_3} - CH(Cl) - C{H_3}$. Thus, it has three carbon atoms and seven hydrogen atoms. Consequently, we are left with ${C_2}{H_5}$ unit from the ether ${C_5}{H_{12}}O$, after getting 2-chloropropane as one of the products. Since ether group is $R - O - R’$, the required R for the given ether will be ${C_2}{H_5}$ and ${R’}$ will be the hydrocarbon unit from 2-chloropropane. Hence, the chemical name of the ether ${C_5}{H_{12}}O$ will be 2-ethoxypropane.
Now, we can write the required chemical equation between given ether, ${C_5}{H_{12}}O$ and $PC{l_5}$ as follows:
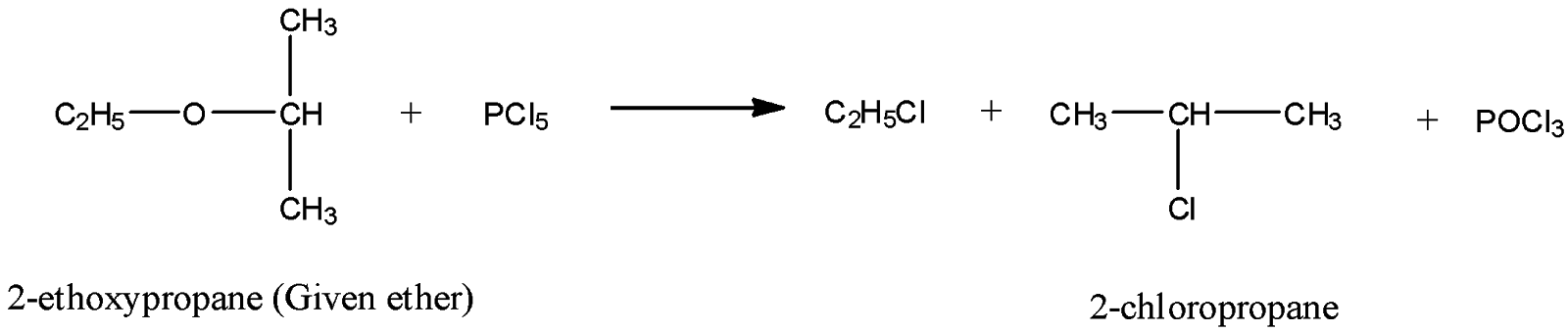
Hence, ${C_5}{H_{12}}O$ (ether) is 2-ethoxypropane.
Thus, option B is the correct answer.
Note: Phosphorus pentachloride, $PC{l_5}$ is used as a chlorinating agent. The reaction of cleavage of ethers refers to the chemical substitution reaction. Due to the higher stability of ethers, the cleavage of the C-O linkage is not done easily. Specific reagents are required for cleavage of ethers and $PC{l_5}$ is that one reagent. It should also be noted from the given chemical reaction in question that dialkyl ethers on reacting with phosphorus pentachloride form alkyl chlorides.
Complete step by step solution:
In the question, it is given that ether linkage ($R-O-R’$) is cleaved by $PC{l_5}$ as follows:
$R - O - R' + PC{l_5} \to RCl + R'Cl + POC{l_3}$
Now, the molecular formula of the given ether in the question is ${C_5}{H_{12}}O$
When ${C_5}{H_{12}}O$ is cleaved by $PC{l_5}$, we get 2-chloropropane as one of the products.
As you know, the molecular formula of 2-chloropropane is: $C{H_3} - CH(Cl) - C{H_3}$. Thus, it has three carbon atoms and seven hydrogen atoms. Consequently, we are left with ${C_2}{H_5}$ unit from the ether ${C_5}{H_{12}}O$, after getting 2-chloropropane as one of the products. Since ether group is $R - O - R’$, the required R for the given ether will be ${C_2}{H_5}$ and ${R’}$ will be the hydrocarbon unit from 2-chloropropane. Hence, the chemical name of the ether ${C_5}{H_{12}}O$ will be 2-ethoxypropane.
Now, we can write the required chemical equation between given ether, ${C_5}{H_{12}}O$ and $PC{l_5}$ as follows:

Hence, ${C_5}{H_{12}}O$ (ether) is 2-ethoxypropane.
Thus, option B is the correct answer.
Note: Phosphorus pentachloride, $PC{l_5}$ is used as a chlorinating agent. The reaction of cleavage of ethers refers to the chemical substitution reaction. Due to the higher stability of ethers, the cleavage of the C-O linkage is not done easily. Specific reagents are required for cleavage of ethers and $PC{l_5}$ is that one reagent. It should also be noted from the given chemical reaction in question that dialkyl ethers on reacting with phosphorus pentachloride form alkyl chlorides.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




