
In the following reaction, \[{\text{C}}{{\text{H}}_3}C{H_2}C{H_2}Br\xrightarrow{{NaCN}}X\xrightarrow{{{H_3}{O^ + }}}Y\xrightarrow[{{H^ + }}]{{C{H_3}C{H_2}OH}}Z\], identify X, Y and Z.
A.
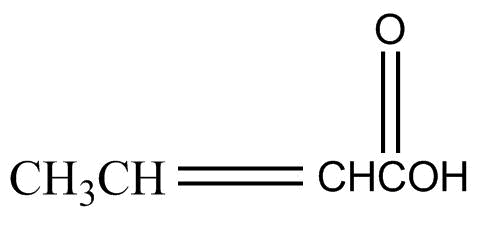
B. \[C{H_3}C{H_2}C{H_2}CH = NOC{H_2}C{H_3}\]
C. \[C{H_3}C{H_2}C{H_2}CH{\left( {OC{H_2}C{H_3}} \right)_2}\]
D.
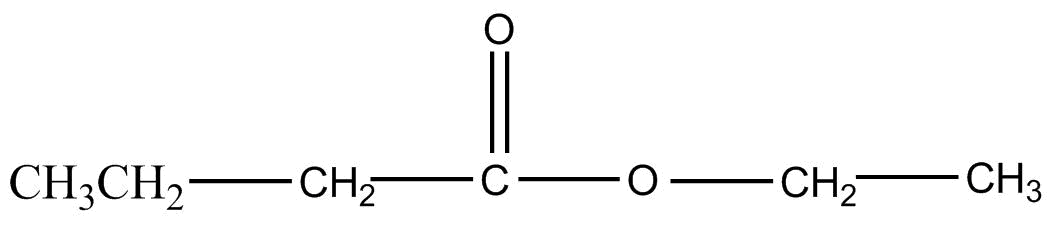
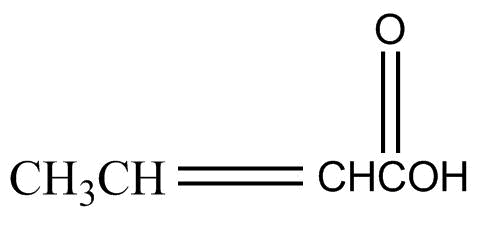

Answer
568.2k+ views
Hint: $C{H_3}C{H_2}CHBr$ is a halo alkane, going under a series of reactions to form an and product. It is a three step reaction involving substitution reaction, hydroxylation in presence of acidic medium and then esterification reaction.
Complete step by step answer: $C{H_3}C{H_2}CHBr$ is an haloalkane going through given steps of reaction.
Step I :- $C{H_3}C{H_2}CHBr$reacting with $NaCN.$ $NaCN$ is present as $N{a^ + }$and $^ - CN.{\;^ - }CN$is a nucleophile that attacks on the carbon of $C{H_3}C{H_2}CHBr$, proceeding with substitution reaction
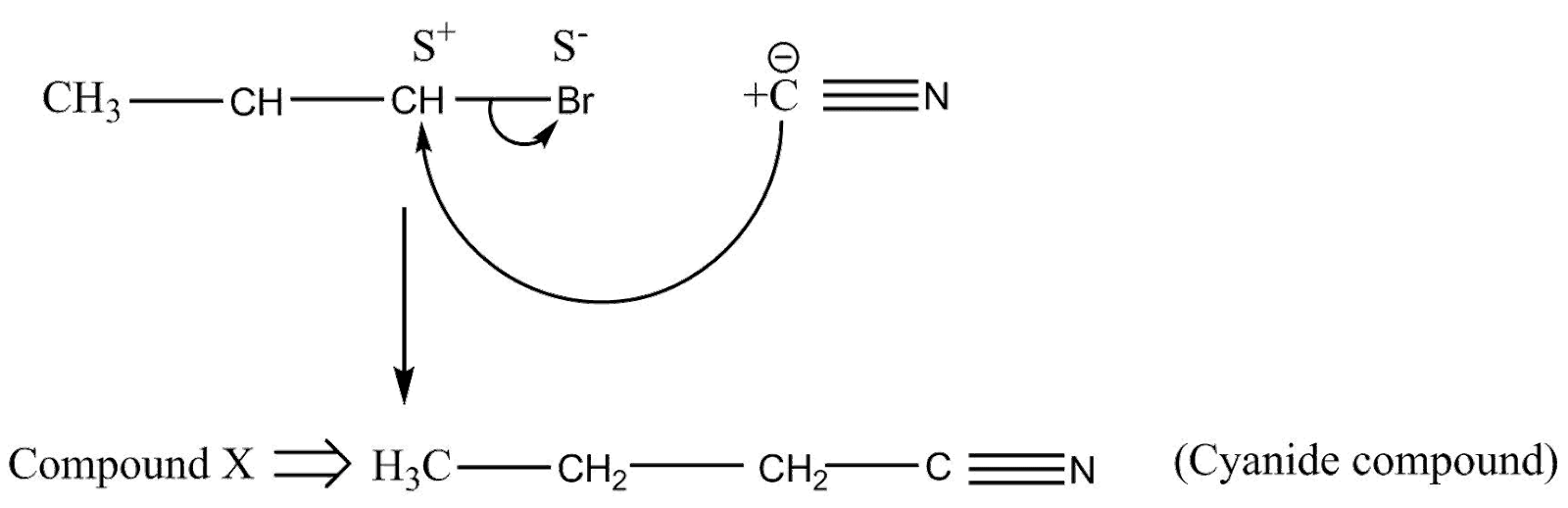
Step II :- when this cyanide compound reacts with ${H_2}{O^ + }$ in presence of heat, it forms a carboxylic acid.
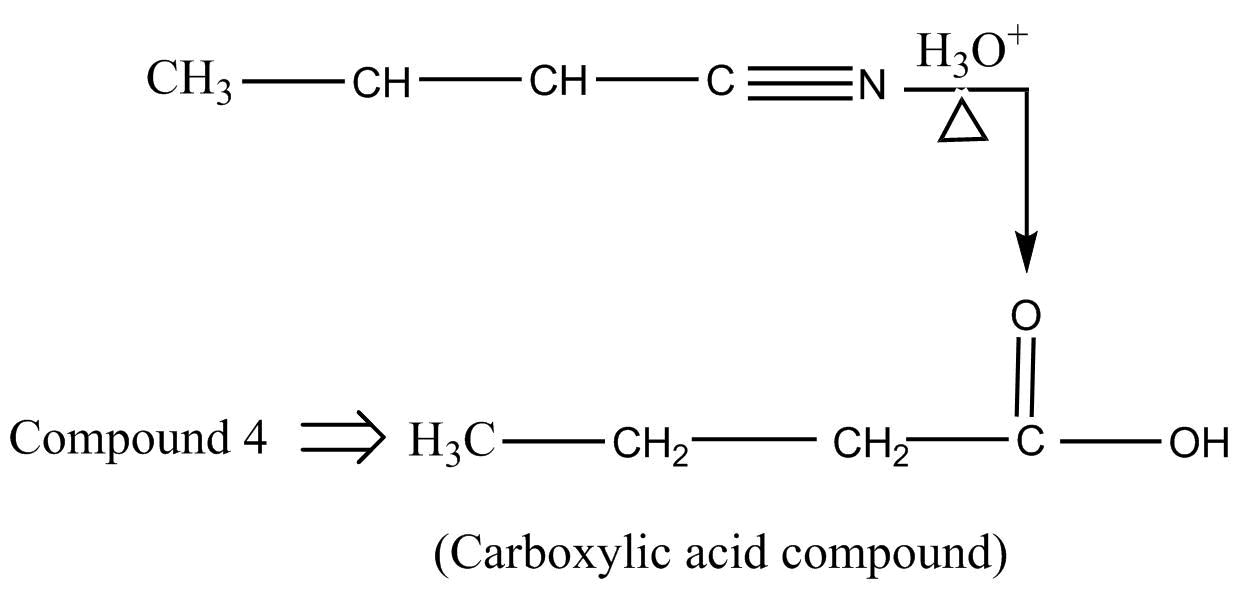
Step III :- Then, this carboxylic acid compound is treated with an alcohol, $C{H_3}C{H_2}OH$ in acidic medium. We know that the reaction of alcohol and carboxylic acid produces an ester by undergoing an esterification reaction.
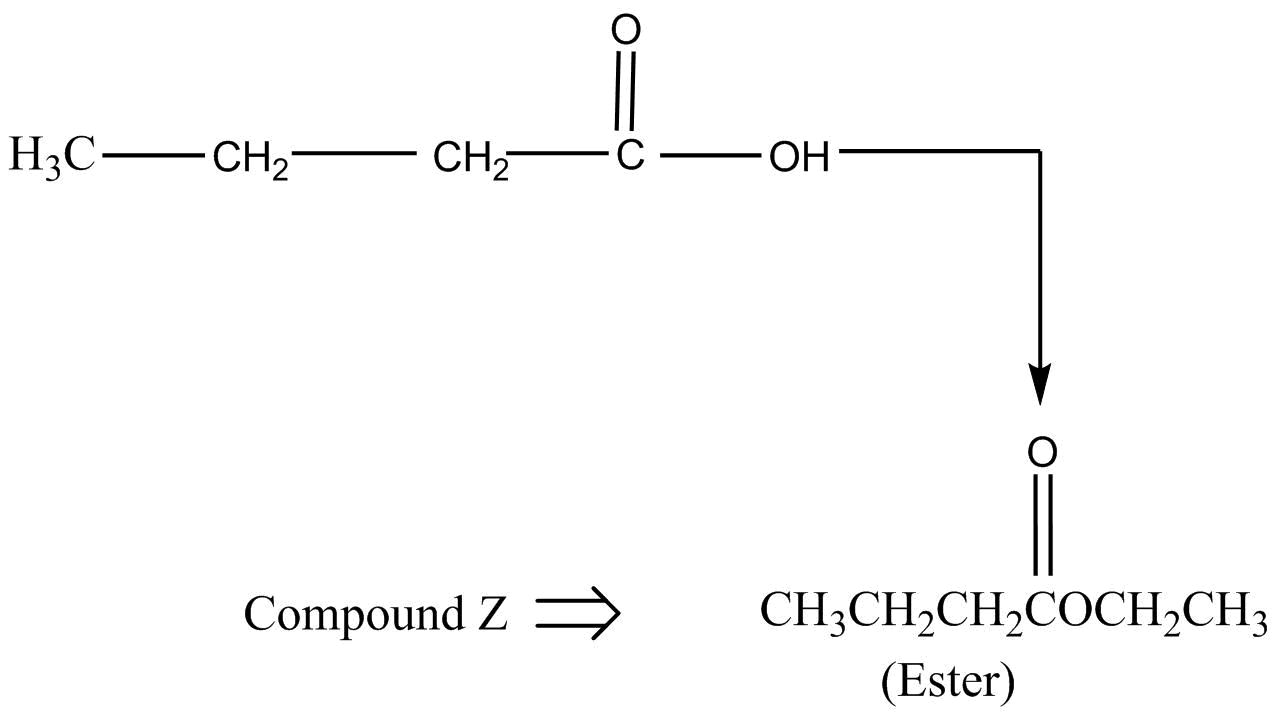
The $IUPAC$ name of compound $z$ is Ethyl but anoate.
Ethyl but anoate is commonly used as an artificial flowering agent similar to orange juice. It is also used in alcoholic beverages like martinis. Ethyl butyrate is one of the most preferred fragrance and flavoring agents.
So, the correct answer is “Option D”.
Note: Esterification reaction in the question took place as shown below
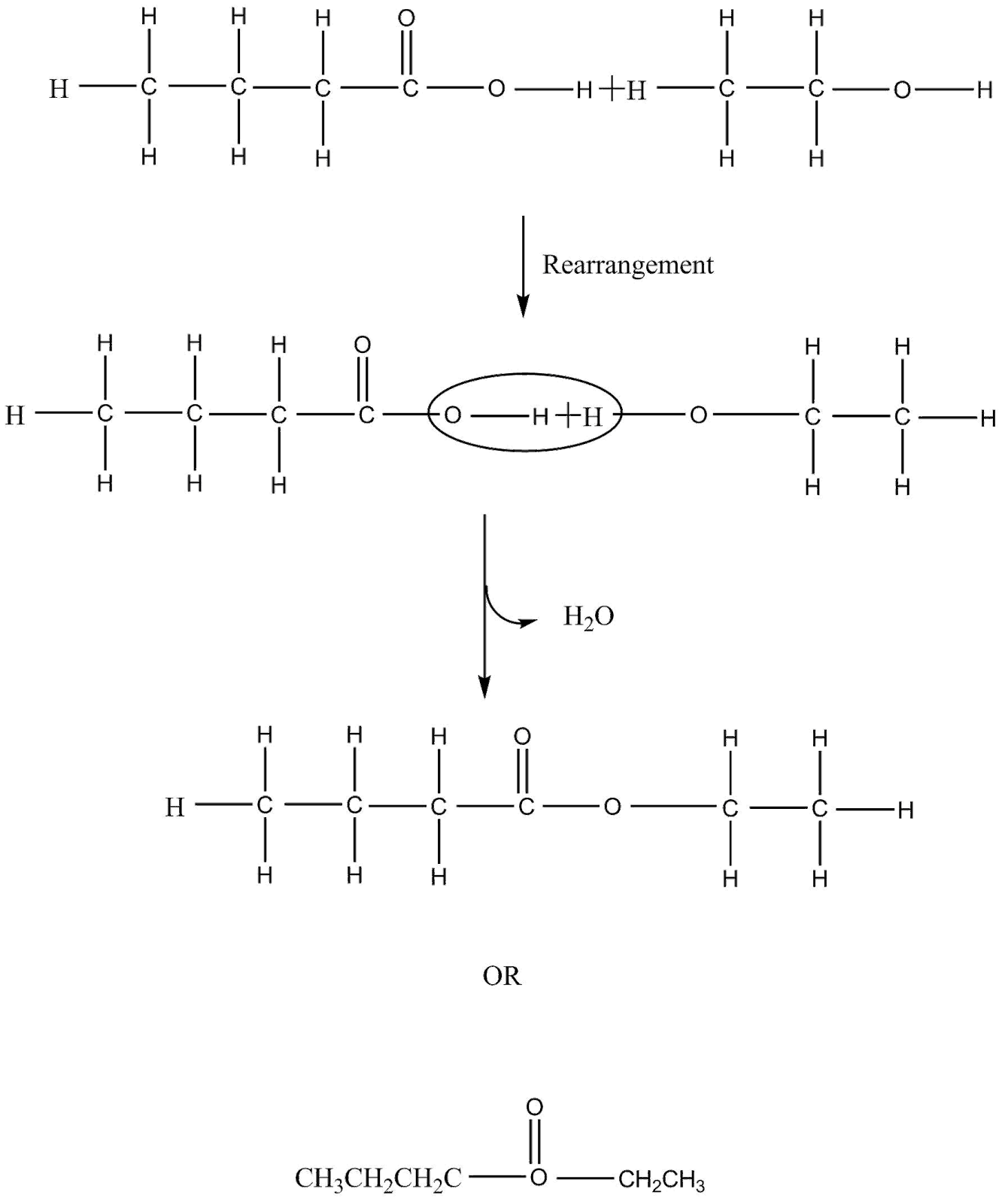
Complete step by step answer: $C{H_3}C{H_2}CHBr$ is an haloalkane going through given steps of reaction.
Step I :- $C{H_3}C{H_2}CHBr$reacting with $NaCN.$ $NaCN$ is present as $N{a^ + }$and $^ - CN.{\;^ - }CN$is a nucleophile that attacks on the carbon of $C{H_3}C{H_2}CHBr$, proceeding with substitution reaction

Step II :- when this cyanide compound reacts with ${H_2}{O^ + }$ in presence of heat, it forms a carboxylic acid.

Step III :- Then, this carboxylic acid compound is treated with an alcohol, $C{H_3}C{H_2}OH$ in acidic medium. We know that the reaction of alcohol and carboxylic acid produces an ester by undergoing an esterification reaction.

The $IUPAC$ name of compound $z$ is Ethyl but anoate.
Ethyl but anoate is commonly used as an artificial flowering agent similar to orange juice. It is also used in alcoholic beverages like martinis. Ethyl butyrate is one of the most preferred fragrance and flavoring agents.
So, the correct answer is “Option D”.
Note: Esterification reaction in the question took place as shown below

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




