
In the following reaction
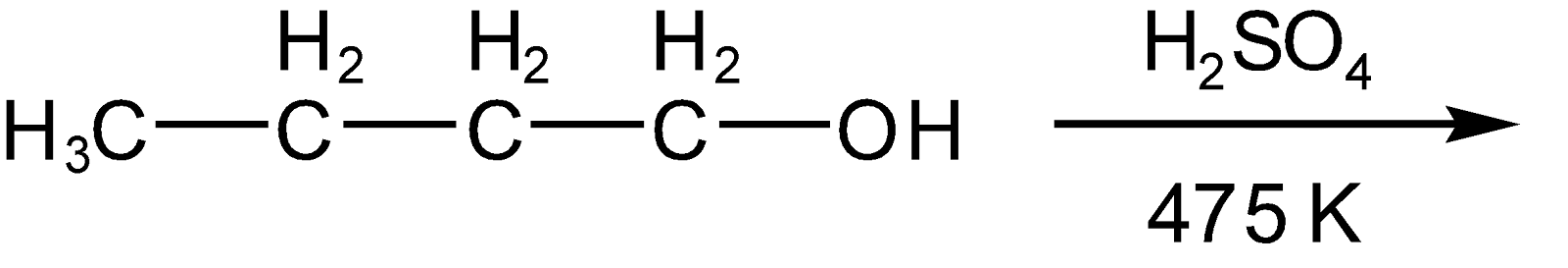
A.
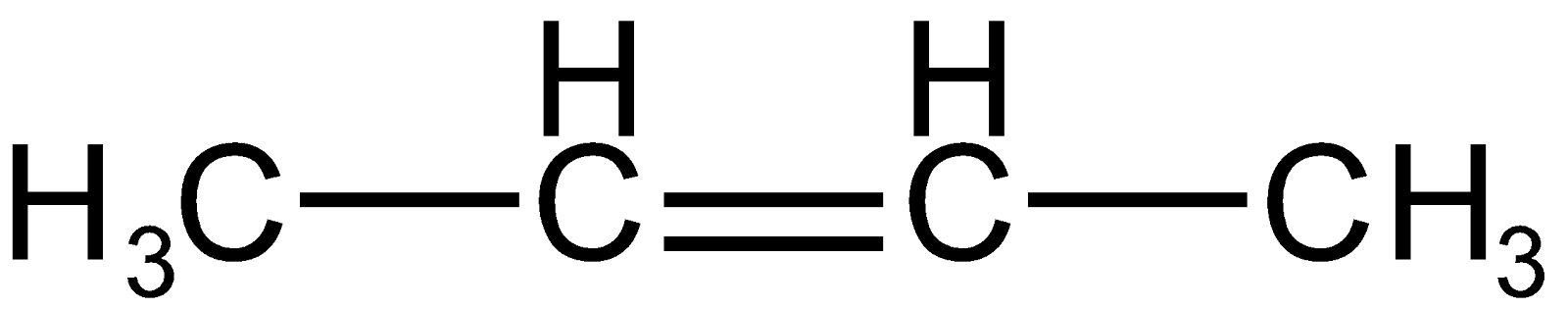 predominant
predominant
B.
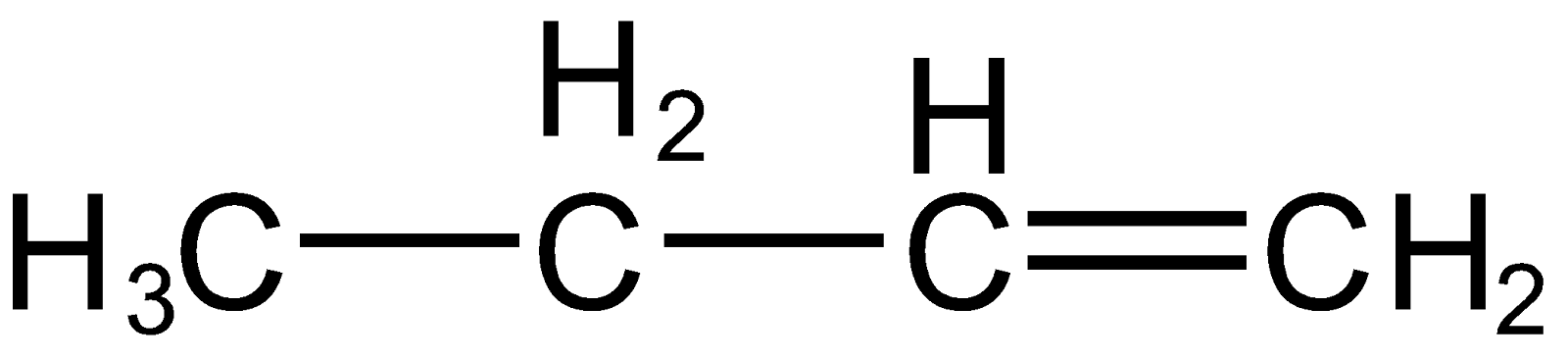 predominant
predominant
C. Both are formed in equal amounts.
D. The amount of production depends on the nature of the catalyst.
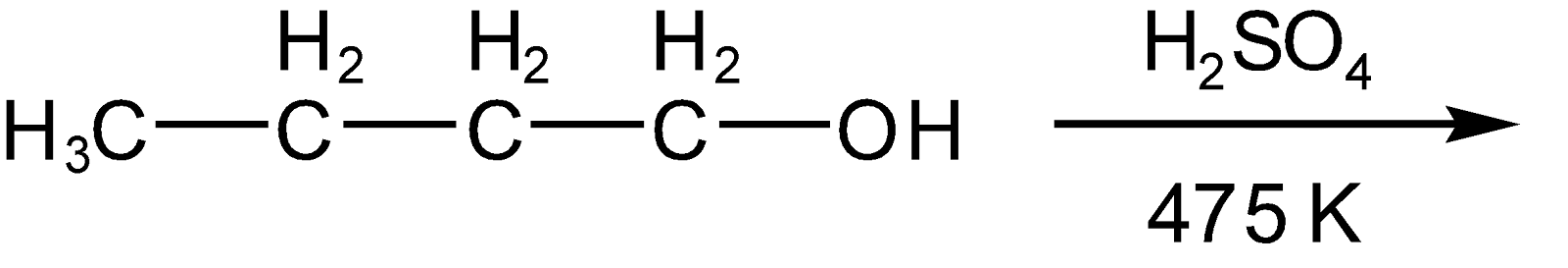
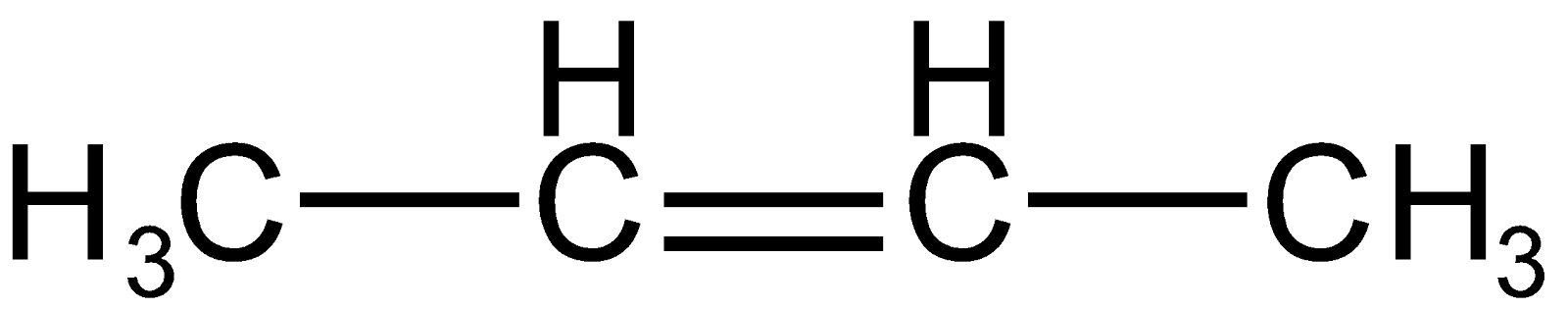
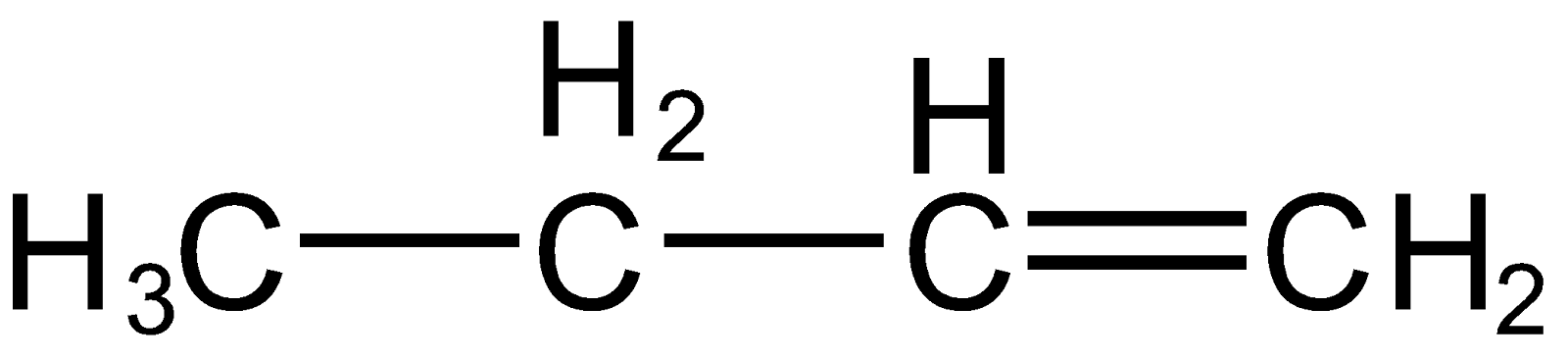
Answer
360.9k+ views
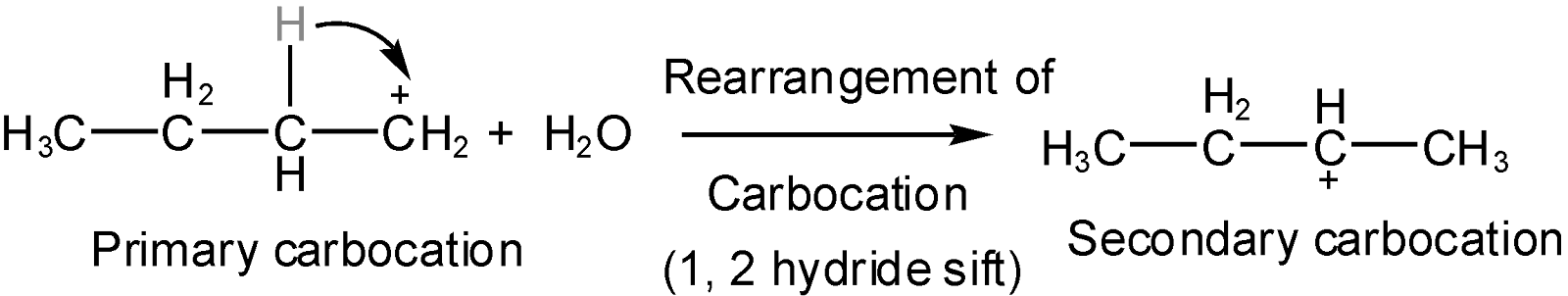
Hint: In the given question reaction will occur in an acidic medium such that OH of the alkyl chain will get protonated to release water molecules and thus carbonation will get generated at the position where OH gets removed. Now the carbonation which is formed is rearranged by 1, 2 hydride shift to get more stable (\[3\text{ }{}^\circ >2{}^\circ >1{}^\circ \]) and the carbonation will shift to beta carbon (alpha carbon at which OH group attached). Now this carbonation will attract the proton of neighboring carbon and two products will form. But the predominant compound will be as per saytzeff's rule.
Complete Step by Step Answer:
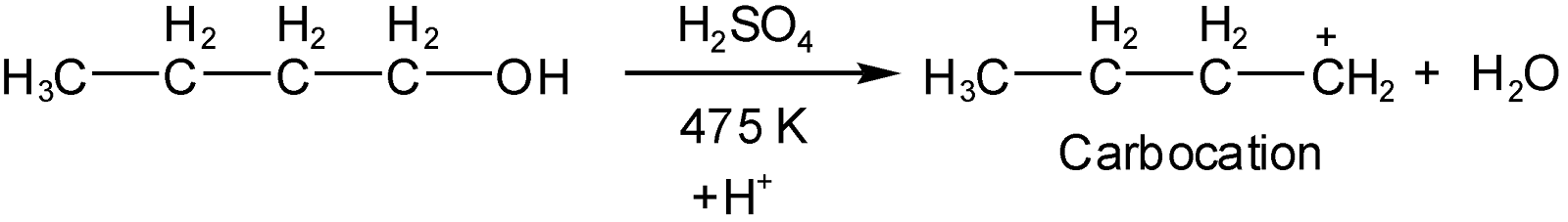
The water molecule eliminate (oxygen taken bond electron from carbon and form bond with \[{{H}^{+}}\]of (\[{{H}_{2}}S{{O}_{4}}\]) as per elimination 1 mechanism (in which carbonation will form as an intermediate) due to this carbonation will generate (carbon with less number of electrons than it’s valence) and as discussed in hint this carbonation will get rearranged on the position of beta carbon to get more stable such as
Now the anion \[HS{{O}_{4}}^{-}\]of acid attacks on the proton of neighboring carbon of carbonation. As there are two carbons in the neighborhood so \[HS{{O}_{4}}^{-}\] can take a proton from the left carbon of carbocation such that the resulting alkene is
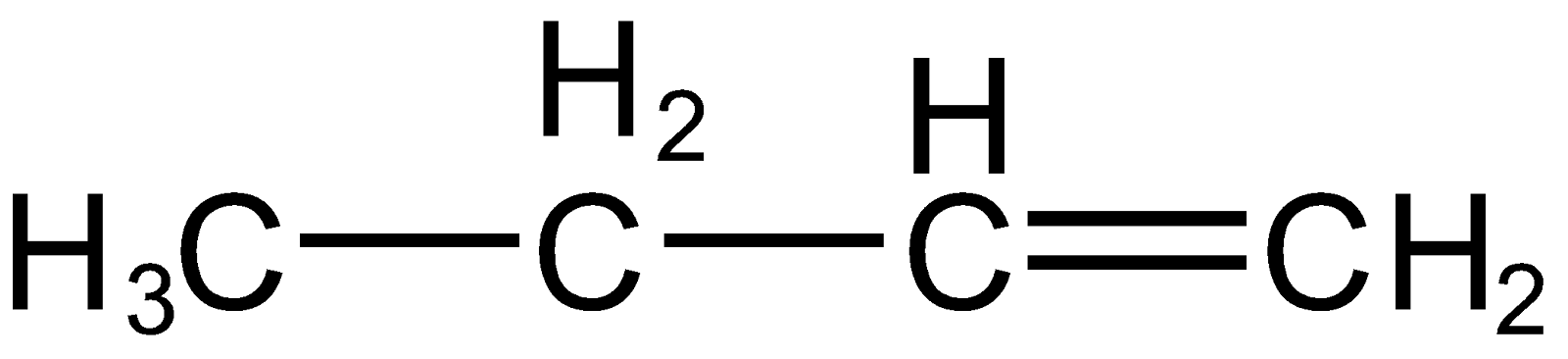
Also, it can take up a proton from the right carbon of carbonation resulting in alkene as
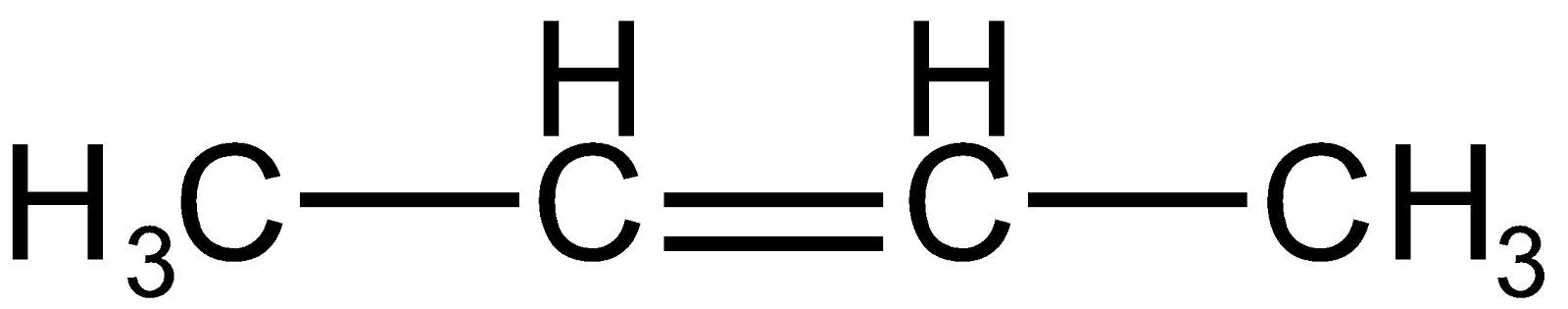
Now as per Saytzeff's rule, more substituted or symmetrical alkene will be the major product of predominate so, the alkene formed by the elimination of proton from the right carbon of carbonation will be major as it is more substituted and also symmetrical.
Thus, the correct option is A.
Note: It is important to note that with the increase in the number of alpha hydrogen stability of the compound increase (hyper conjugation). The alkene formed first (
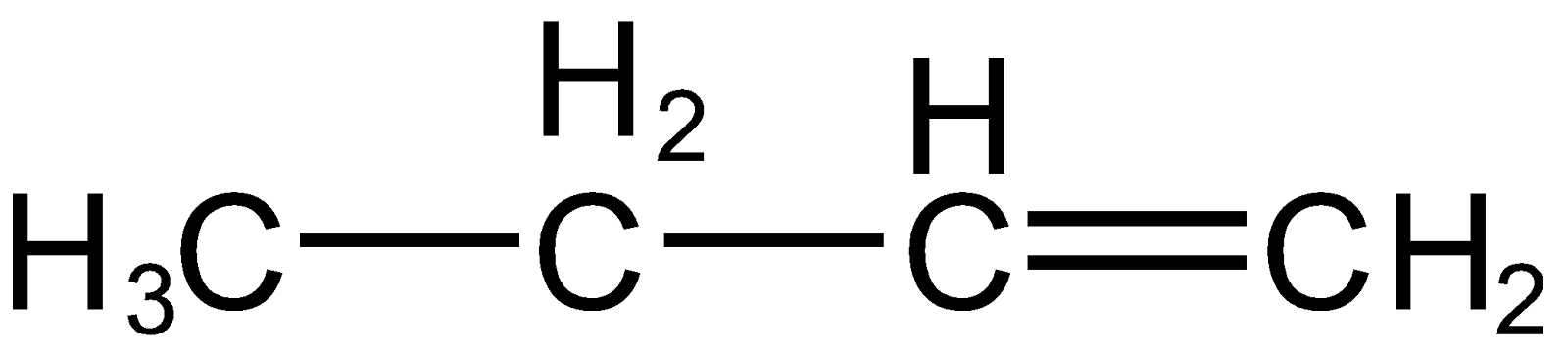 ) has only two alpha hydrogen (hydrogen present on carbon which is next to double bonded carbon) whereas the other alkene (
) has only two alpha hydrogen (hydrogen present on carbon which is next to double bonded carbon) whereas the other alkene (
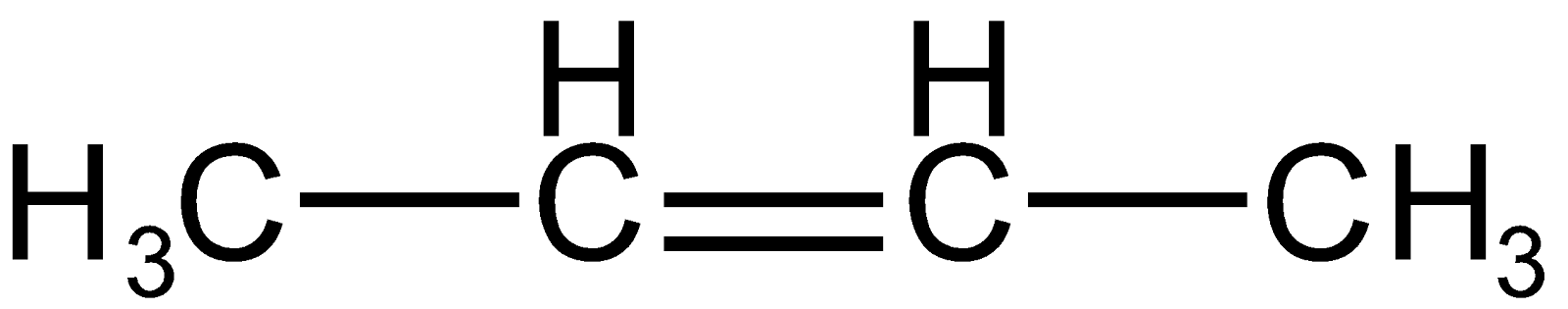 ) has 6 alpha hydrogen so, the latter alkene is more stable thus it will be predominant.
) has 6 alpha hydrogen so, the latter alkene is more stable thus it will be predominant.
Complete Step by Step Answer:
The water molecule eliminate (oxygen taken bond electron from carbon and form bond with \[{{H}^{+}}\]of (\[{{H}_{2}}S{{O}_{4}}\]) as per elimination 1 mechanism (in which carbonation will form as an intermediate) due to this carbonation will generate (carbon with less number of electrons than it’s valence) and as discussed in hint this carbonation will get rearranged on the position of beta carbon to get more stable such as


Now the anion \[HS{{O}_{4}}^{-}\]of acid attacks on the proton of neighboring carbon of carbonation. As there are two carbons in the neighborhood so \[HS{{O}_{4}}^{-}\] can take a proton from the left carbon of carbocation such that the resulting alkene is
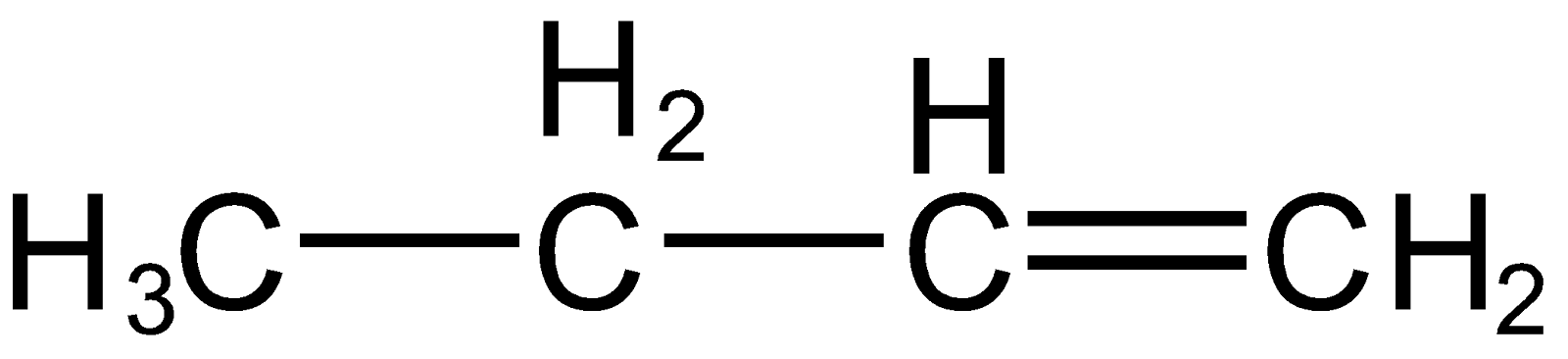
Also, it can take up a proton from the right carbon of carbonation resulting in alkene as
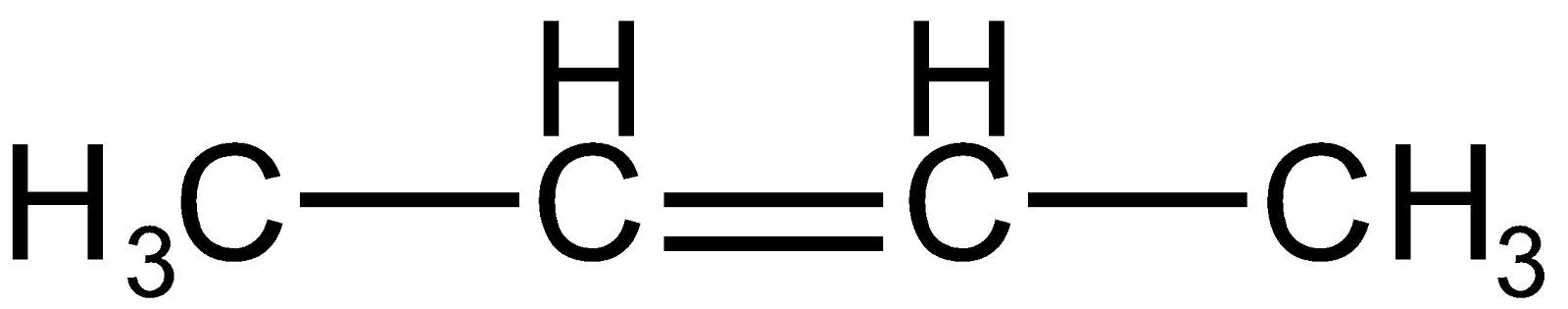
Now as per Saytzeff's rule, more substituted or symmetrical alkene will be the major product of predominate so, the alkene formed by the elimination of proton from the right carbon of carbonation will be major as it is more substituted and also symmetrical.
Thus, the correct option is A.
Note: It is important to note that with the increase in the number of alpha hydrogen stability of the compound increase (hyper conjugation). The alkene formed first (
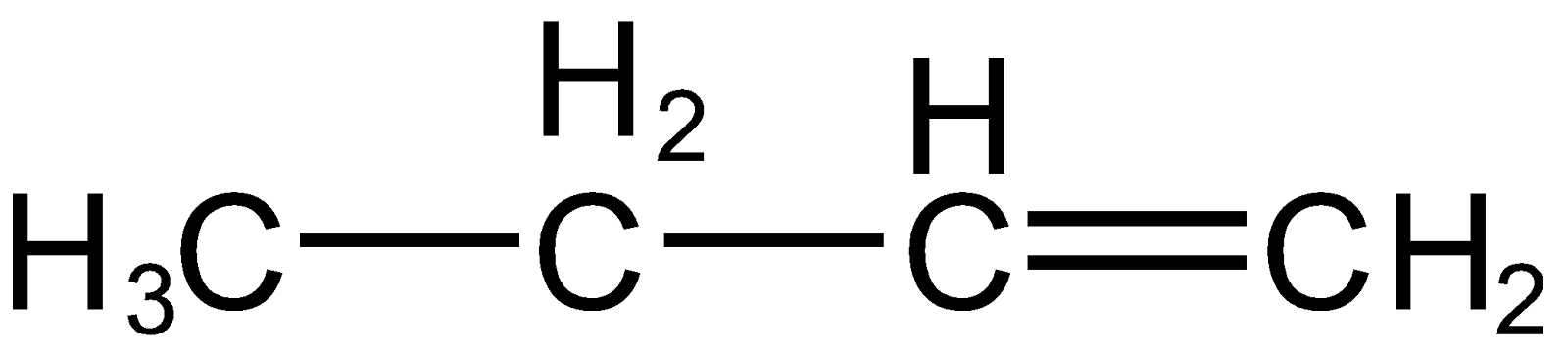
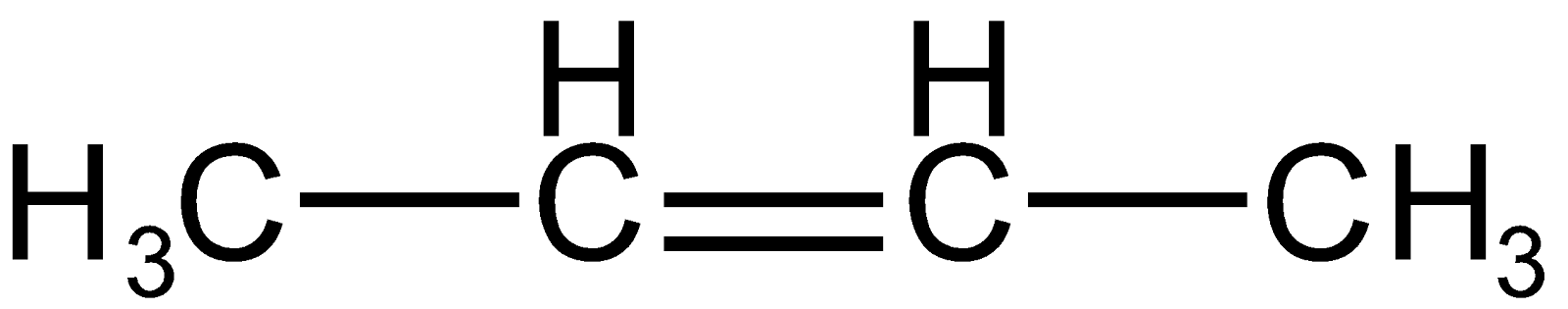
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
What are the factors of 100 class 7 maths CBSE

Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Difference Between Plant Cell and Animal Cell




