
In the following compound, the number of sp hybridized carbons is:
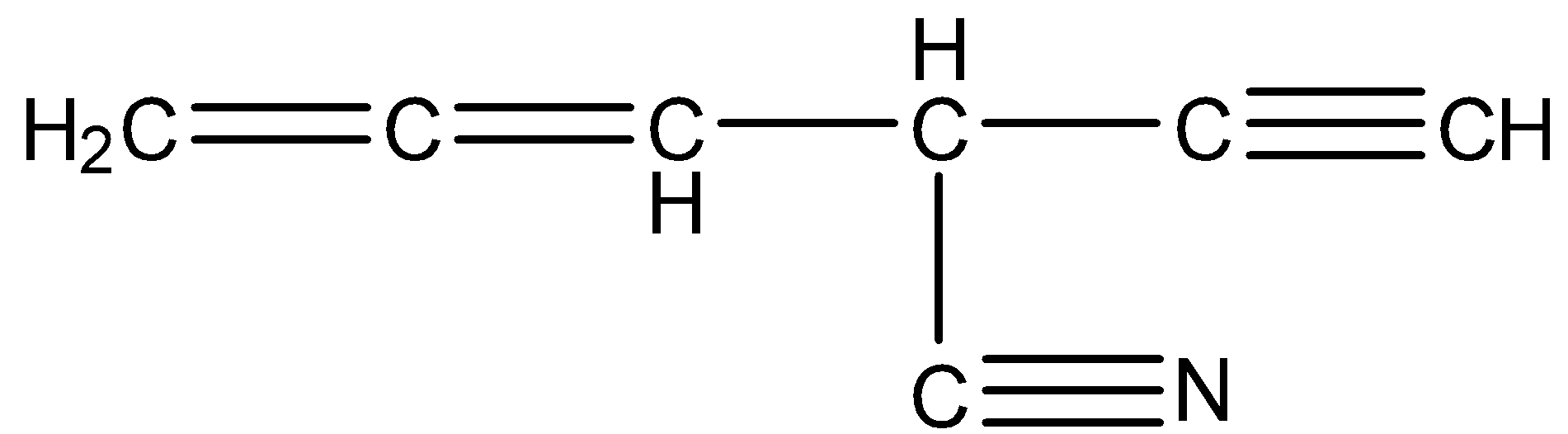
A. 2
B. 3
C. 4
D. 5

Answer
592.2k+ views
Hint: If a carbon is \[s{{p}^{3}}\] hybridized it contains four sigma bonds.
If a carbon is \[s{{p}^{2}}\] hybridized it contains three sigma bonds and one pi bond.
If a carbon is \[sp\] hybridized it contains two sigma bonds and two pi bonds.
Based on the above statements we can easily find the hybridization of carbons in a given molecule.
Complete step by step answer:
The structure of the given compound is
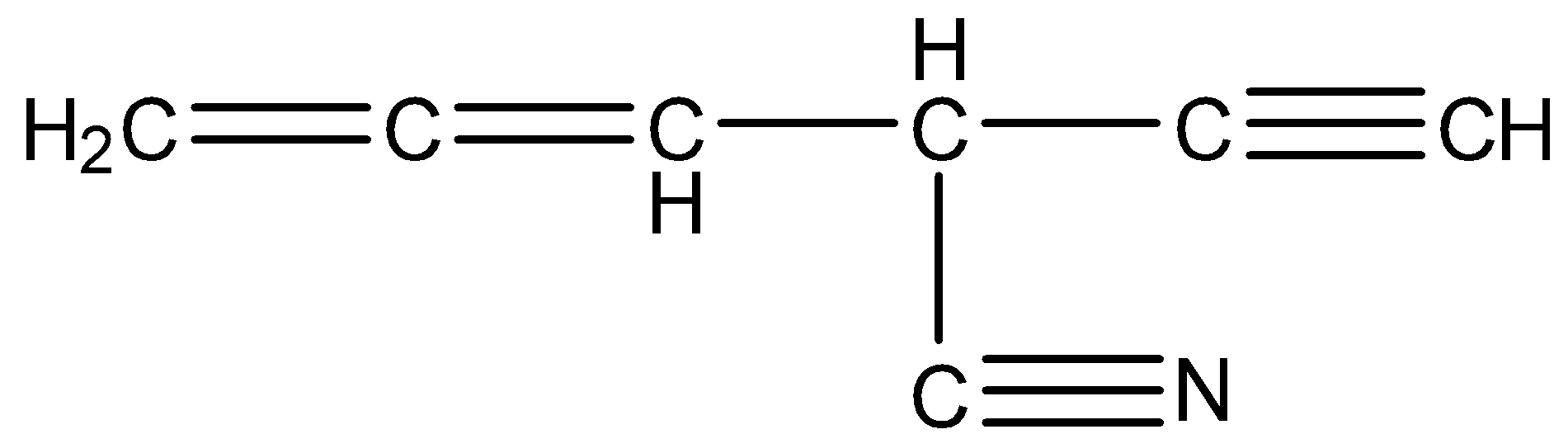
The number of carbons present in the given compound is 7.
Now we have to give numbers to all the carbons.

Coming to carbon number 1, it formed two sigma bonds (one with Hydrogen and the other with carbon-2) and two pi bonds (with carbon 2). So, carbon 1 is sp hybridized.
Coming to carbon number 2, it formed two sigma bonds (one sigma bond with carbon one and second sigma bond with carbon 3) and two pi bonds (with carbon 1). So, carbon 2 is sp hybridized.
Coming to carbon number 3, it formed four sigma bonds (one with carbon 2, second sigma bond with carbon 4, third sigma bond with carbon 7 and fourth sigma bond with hydrogen). So, carbon 3 is \[s{{p}^{3}}\] hybridized.
Coming to carbon number 4, it formed three sigma bonds (with carbon 3, with carbon 5 and with hydrogen) and one pi bond (with carbon 5). So, carbon 4 is\[s{{p}^{2}}\] hybridized.
Coming to carbon 5, it formed two sigma bonds (with carob 4 and with carbon 6) and two pi bonds (with carbon 4 and with carbon 5). So, carbon 5 is sp hybridized.
Coming to carbon 6, it formed three sigma bonds (with carbon 5 and with two hydrogens) and one pi bond (with carbon 5). So, carbon 6 is \[s{{p}^{2}}\] hybridized.
Coming to carbon 7. It formed two sigma bonds (with carbon 3 and with nitrogen) and two pi bonds (with nitrogen). So, carbon 7 is sp hybridized.
Therefore there are four sp hybridized carbons (carbon number 1, 2, 5, and 7) in the given compound.
So, the correct option is C.
Note: In a double bond the type of bonds present are one sigma bond and one pi bond, in triple bond the types of bonds present are one sigma bond and two pi bonds. Sigma bond is going to form through axial overlapping of orbitals and pi bond is going to form through sidewise overlapping of orbitals.
If a carbon is \[s{{p}^{2}}\] hybridized it contains three sigma bonds and one pi bond.
If a carbon is \[sp\] hybridized it contains two sigma bonds and two pi bonds.
Based on the above statements we can easily find the hybridization of carbons in a given molecule.
Complete step by step answer:
The structure of the given compound is

The number of carbons present in the given compound is 7.
Now we have to give numbers to all the carbons.

Coming to carbon number 1, it formed two sigma bonds (one with Hydrogen and the other with carbon-2) and two pi bonds (with carbon 2). So, carbon 1 is sp hybridized.
Coming to carbon number 2, it formed two sigma bonds (one sigma bond with carbon one and second sigma bond with carbon 3) and two pi bonds (with carbon 1). So, carbon 2 is sp hybridized.
Coming to carbon number 3, it formed four sigma bonds (one with carbon 2, second sigma bond with carbon 4, third sigma bond with carbon 7 and fourth sigma bond with hydrogen). So, carbon 3 is \[s{{p}^{3}}\] hybridized.
Coming to carbon number 4, it formed three sigma bonds (with carbon 3, with carbon 5 and with hydrogen) and one pi bond (with carbon 5). So, carbon 4 is\[s{{p}^{2}}\] hybridized.
Coming to carbon 5, it formed two sigma bonds (with carob 4 and with carbon 6) and two pi bonds (with carbon 4 and with carbon 5). So, carbon 5 is sp hybridized.
Coming to carbon 6, it formed three sigma bonds (with carbon 5 and with two hydrogens) and one pi bond (with carbon 5). So, carbon 6 is \[s{{p}^{2}}\] hybridized.
Coming to carbon 7. It formed two sigma bonds (with carbon 3 and with nitrogen) and two pi bonds (with nitrogen). So, carbon 7 is sp hybridized.
Therefore there are four sp hybridized carbons (carbon number 1, 2, 5, and 7) in the given compound.
So, the correct option is C.
Note: In a double bond the type of bonds present are one sigma bond and one pi bond, in triple bond the types of bonds present are one sigma bond and two pi bonds. Sigma bond is going to form through axial overlapping of orbitals and pi bond is going to form through sidewise overlapping of orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




