
In the dichromate di-anion:
(A) 4 $Cr-O$ bonds are equivalent
(B) 6 $Cr-O$ bonds are equivalent
(C) All $Cr-O$ bonds are equivalent
(D) All $Cr-O$ bonds are non-equivalent
Answer
582.6k+ views
Hint: The dichromate di-anion has two chromium atoms and seven oxygen atoms. Each chromium atom is attached to three oxygen atoms and one oxygen atom forms a bridge between these chromium atoms.
Complete step by step solution:
The dichromate di-anion means there are two chromate ions and there is $-2$ charge on the overall molecule. The dichromate di-anion has two chromium atoms and seven oxygen atoms. The formula of dichromate di-anion is $C{{r}_{2}}O_{7}^{2-}$. It is also known as dichromate, dichromate ion, bichromate, etc. The molecular mass of dichromate ions is $215.99\text{ g/mol}$.
So, there are two chromium atoms in the dichromate ion, and each chromium atom is joined to three oxygen atoms. This means that there are six $Cr-O$ independent bonds. And both these molecules are joined by the same oxygen atom which acts as a bridge. This molecule has a -2 charge on the overall molecule which is because of the charge on two oxygen atoms. Now due to resonance, the six $Cr-O$ bonds have equal length and the charge is on the overall molecule. The bond length of the $Cr-O$ bond lies between the length of a single bond and the length of a double bond.
The structure of the dichromate di-anion can be represented in two forms. These are given below:
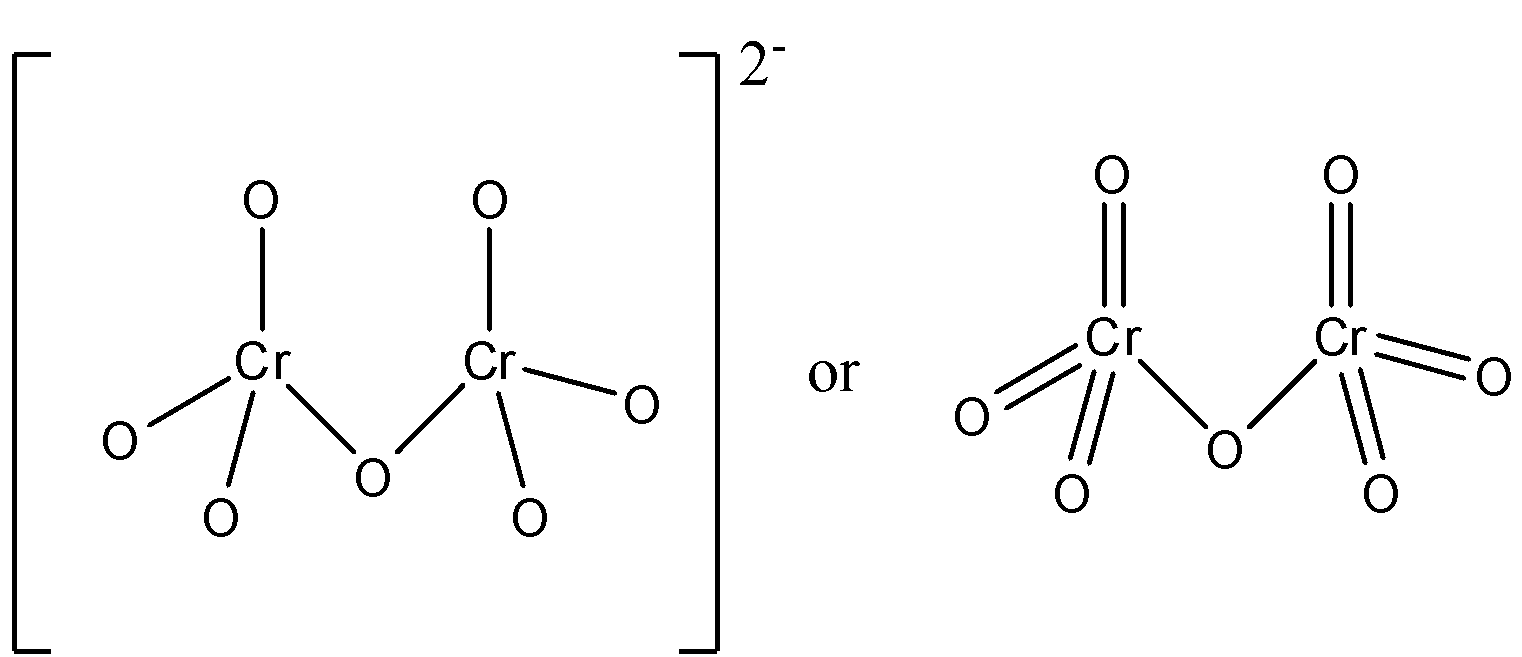
Therefore, the correct answer is an option (B)- 6 $Cr-O$ bonds are equivalent.
Note: The formal charge on the dichromate ion is -2. The exact mass of dichromate ion is $215.85\text{ g/mol}$. Dichromate ion is basic because it can accept hydrogen ions.
Complete step by step solution:
The dichromate di-anion means there are two chromate ions and there is $-2$ charge on the overall molecule. The dichromate di-anion has two chromium atoms and seven oxygen atoms. The formula of dichromate di-anion is $C{{r}_{2}}O_{7}^{2-}$. It is also known as dichromate, dichromate ion, bichromate, etc. The molecular mass of dichromate ions is $215.99\text{ g/mol}$.
So, there are two chromium atoms in the dichromate ion, and each chromium atom is joined to three oxygen atoms. This means that there are six $Cr-O$ independent bonds. And both these molecules are joined by the same oxygen atom which acts as a bridge. This molecule has a -2 charge on the overall molecule which is because of the charge on two oxygen atoms. Now due to resonance, the six $Cr-O$ bonds have equal length and the charge is on the overall molecule. The bond length of the $Cr-O$ bond lies between the length of a single bond and the length of a double bond.
The structure of the dichromate di-anion can be represented in two forms. These are given below:

Therefore, the correct answer is an option (B)- 6 $Cr-O$ bonds are equivalent.
Note: The formal charge on the dichromate ion is -2. The exact mass of dichromate ion is $215.85\text{ g/mol}$. Dichromate ion is basic because it can accept hydrogen ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




