
In the conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as Na or K) in liquid $N{{H}_{3}}$ and alcohol (MeOH or EtOH). What is the mechanism taking place in the formation of intermediate species in the following sequence
\[R-\equiv -R{{\xrightarrow[+EtOH]{Na+liq.N{{H}_{3}}}}_{H}}^{R}\rangle ={{\langle }_{R}}^{H}\]
(A) Radical anion $\to $ vinylic radical $\to $ trans-vinylic anion $\to $ trans alkene
(B) Radical anion $\to $ trans-vinylic anion $\to $ vinylic radical $\to $ trans alkene
(C) Vinylic radical $\to $ Radical anion $\to $ trans-vinylic anion $\to $ trans alkene
(D) Vinylic radical $\to $ trans-vinylic anion $\to $ radical anion $\to $ trans alkene
Answer
577.2k+ views
Hint: The conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as Na or K) in liquid $N{{H}_{3}}$ and alcohol (MeOH or EtOH), four radicals are formed i.e. radical anion, vinylic radical, trans-vinylic anion, trans alkene.
Complete step by step solution:
Let us first discuss the Birch reduction-
-The Birch reduction is an organic chemical reaction where aromatic compounds which have a benzenoid ring are converted into 1,4-cyclohexadiene which have two hydrogen atoms attached opposite ends of the molecule. It is a very useful reaction in synthetic organic chemistry.
-Conjugated enamines can also be formed from the Birch reduction of aniline. Alkynes can also undergo Birch reduction to form alkenes.
-Birch reduction is named after the Australian chemist Arthur Birch. The reaction type involved in this process is organic redox reaction.
-Alkynes are selectively converted into trans alkenes when they are reduced by a solution of sodium (or lithium) in liquid that contains stoichiometric amounts of an alcohol such as ethanol.
-The first step of this reduction is one-electron transfer into an antibonding $\pi $ orbital of alkyne, which yields a radical anion.
-Subsequently, protonation of the radical anion, an additional one-electron transfer, and a concluding protonation yield a trans alkene.
Illustration- The mechanism:
-Reduction of the alkyne by sodium results in breakage of the C-C double bond and formation of an anion adjacent to the radical.
-The radical that is formed can interconvert between its cis and trans form, but the trans is generally more stable due to steric factors. The anion is then protonated by $N{{H}_{3}}$ (the only acid present in solution) to give the vinyl radical, which is then reduced by a second equivalent of Na to give a second anion. This is then converted to the alkene by protonation with the second equivalent of $N{{H}_{3}}$. So, the net process gives a trans alkene and the two equivalents of $NaN{{H}_{2}}$.
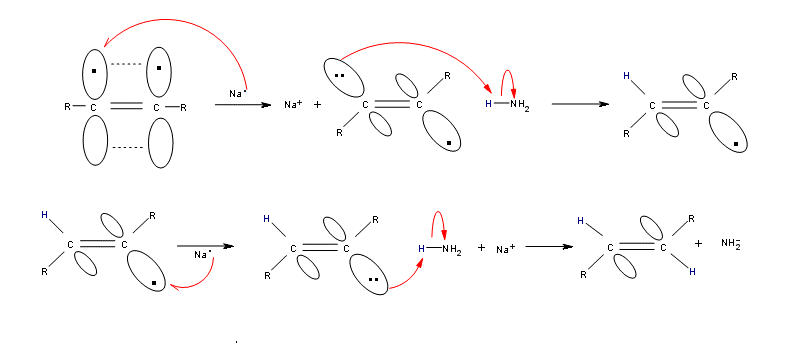
Therefore, option (A) Radical anion $\to $ vinylic radical $\to $ trans-vinylic anion $\to $ trans alkene is correct.
Note: Do not get confused between vinylic radical and vinylic anion. Always remember that the radical is formed prior to the anion formation.
Complete step by step solution:
Let us first discuss the Birch reduction-
-The Birch reduction is an organic chemical reaction where aromatic compounds which have a benzenoid ring are converted into 1,4-cyclohexadiene which have two hydrogen atoms attached opposite ends of the molecule. It is a very useful reaction in synthetic organic chemistry.
-Conjugated enamines can also be formed from the Birch reduction of aniline. Alkynes can also undergo Birch reduction to form alkenes.
-Birch reduction is named after the Australian chemist Arthur Birch. The reaction type involved in this process is organic redox reaction.
-Alkynes are selectively converted into trans alkenes when they are reduced by a solution of sodium (or lithium) in liquid that contains stoichiometric amounts of an alcohol such as ethanol.
-The first step of this reduction is one-electron transfer into an antibonding $\pi $ orbital of alkyne, which yields a radical anion.
-Subsequently, protonation of the radical anion, an additional one-electron transfer, and a concluding protonation yield a trans alkene.
Illustration- The mechanism:
-Reduction of the alkyne by sodium results in breakage of the C-C double bond and formation of an anion adjacent to the radical.
-The radical that is formed can interconvert between its cis and trans form, but the trans is generally more stable due to steric factors. The anion is then protonated by $N{{H}_{3}}$ (the only acid present in solution) to give the vinyl radical, which is then reduced by a second equivalent of Na to give a second anion. This is then converted to the alkene by protonation with the second equivalent of $N{{H}_{3}}$. So, the net process gives a trans alkene and the two equivalents of $NaN{{H}_{2}}$.

Therefore, option (A) Radical anion $\to $ vinylic radical $\to $ trans-vinylic anion $\to $ trans alkene is correct.
Note: Do not get confused between vinylic radical and vinylic anion. Always remember that the radical is formed prior to the anion formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




