
In $P{O_4}^{3 - }$, the formal charge on each oxygen atom, and ${\text{P - O}}$ bond order respectively are:
(a) $ - 0 \cdot 75,0 \cdot 6$
(b) $ - 0 \cdot 75,1 \cdot 0$
(c) $ - 0 \cdot 75,1 \cdot 25$
(d) $ - 3,1 \cdot 25$
Answer
585.6k+ views
Hint: Formal charge calculating equation is a formal way of comparing the total number of valence electron in a neutral, and isolated atom with the number of valence electrons around the atom in the molecule in which it is bonded, and order is the number of bonds between a pair of atoms.
Complete step by step answer:
Step (1): Calculation of formal charge:
Formal charge = $\left( {{\text{Number of valence electrons in the neutral isolated atom}}} \right)$ - $\left( {{\text{Number of valence electrons around the atom in the bonded state with the molecule}}} \right)$
= $\left( {{\text{Number of valence electrons in the neutral isolated atom}}} \right)$- \[\dfrac{1}{2}\left( {{\text{Number of shared electrons in the covalent bond between them}}} \right)\] -$\left( {{\text{Number of lone pair of electrons}}} \right)$ ..……… $\left( 1 \right)$
Valence electrons correspond to the group number of the atom in the periodic table.
Lone pair electrons are the group of two electrons in a pair which are left on the atom after the bond formation.
Shared electrons are the electrons which took part in the bond formation. $\dfrac{1}{2}$ is used in the formula because each bond consists of two electrons, so, each atom can get the credit of one electron only in a bond.
The structure of $P{O_4}^{3 - }$ will be:
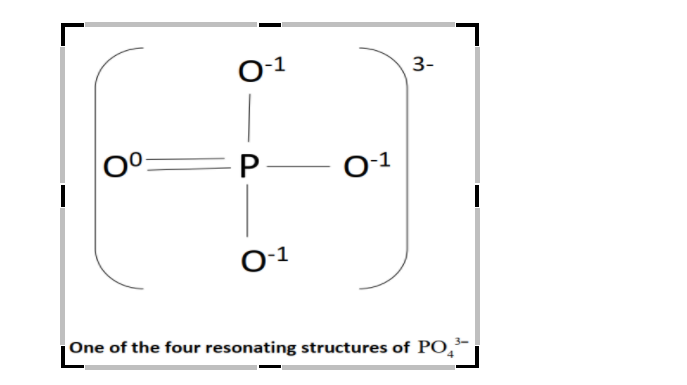
Since, formal charge is the charge assigned to an atom in the molecule, so, the numbers above the atoms signifies their formal charge in the above figure.
Formal charge of ${\text{O - atom}}$having single bond with ${\text{P - atom}}$from equation $\left( 1 \right)$ will be:
= $\left( {{\text{Number of valence electrons in the O - atom}}} \right)$-\[\dfrac{1}{2}\left( {{\text{Number of shared electrons in the covalent bond between O and P}}} \right)\]- $\left( {{\text{Number of lone pair of electrons of O - atom}}} \right)$
= $6 - \dfrac{1}{2} \times 2 - 6$
$ = - 1$
Formal charge of ${\text{O - atom}}$having single bond with ${\text{P - atom}}$from equation $\left( 1 \right)$ will be:
= $6 - \dfrac{1}{2} \times 4 - 6$
${\text{ = 0}}$
Since, $P{O_4}^{3 - }$ has ${\text{4}}$ resonating structures, so, formal charge will be divided equally between the ${\text{3}}$ single bonded ${\text{O - atoms}}$.
Each atom has charge = ${\text{ - 1}}$. So, ${\text{3}}$ atoms have charge = $ - 1 \times 3 = - 3$.
Total number of ${\text{O - atoms}}$ present = ${\text{4}}$. So, Formal charge of each ${\text{O - atom}}$
= $\dfrac{{{\text{Total charge}}}}{{{\text{Total atoms}}}}$
= $\dfrac{{ - 3}}{4}$
$ = - 0 \cdot 75$
Step (2): Calculation of ${\text{P - O}}$ bond order:
Total number of bonds in $P{O_4}^{3 - }$ = ${\text{5}}$, and Total number of resonating structures of $P{O_4}^{3 - }$ = ${\text{4}}$
Now, Bond order = $\dfrac{{{\text{Total number of bonds in the molecule}}}}{{{\text{Total number of resonating structures}}}}$
= $\dfrac{5}{4}$
= $1 \cdot 25$
So, the formal charge on each ${\text{O - atom}}$ is $ - 0 \cdot 75$, and ${\text{P - O}}$ bond order is $1 \cdot 25$.
So, the correct answer is Option C .
Note:
In the formula of Formal charge, do not write the number of pairs of electrons, instead write the total number of electrons in each pair. Also, the bond order is inversely proportional to the bond length between the atoms in consideration.
Complete step by step answer:
Step (1): Calculation of formal charge:
Formal charge = $\left( {{\text{Number of valence electrons in the neutral isolated atom}}} \right)$ - $\left( {{\text{Number of valence electrons around the atom in the bonded state with the molecule}}} \right)$
= $\left( {{\text{Number of valence electrons in the neutral isolated atom}}} \right)$- \[\dfrac{1}{2}\left( {{\text{Number of shared electrons in the covalent bond between them}}} \right)\] -$\left( {{\text{Number of lone pair of electrons}}} \right)$ ..……… $\left( 1 \right)$
Valence electrons correspond to the group number of the atom in the periodic table.
Lone pair electrons are the group of two electrons in a pair which are left on the atom after the bond formation.
Shared electrons are the electrons which took part in the bond formation. $\dfrac{1}{2}$ is used in the formula because each bond consists of two electrons, so, each atom can get the credit of one electron only in a bond.
The structure of $P{O_4}^{3 - }$ will be:

Since, formal charge is the charge assigned to an atom in the molecule, so, the numbers above the atoms signifies their formal charge in the above figure.
Formal charge of ${\text{O - atom}}$having single bond with ${\text{P - atom}}$from equation $\left( 1 \right)$ will be:
= $\left( {{\text{Number of valence electrons in the O - atom}}} \right)$-\[\dfrac{1}{2}\left( {{\text{Number of shared electrons in the covalent bond between O and P}}} \right)\]- $\left( {{\text{Number of lone pair of electrons of O - atom}}} \right)$
= $6 - \dfrac{1}{2} \times 2 - 6$
$ = - 1$
Formal charge of ${\text{O - atom}}$having single bond with ${\text{P - atom}}$from equation $\left( 1 \right)$ will be:
= $6 - \dfrac{1}{2} \times 4 - 6$
${\text{ = 0}}$
Since, $P{O_4}^{3 - }$ has ${\text{4}}$ resonating structures, so, formal charge will be divided equally between the ${\text{3}}$ single bonded ${\text{O - atoms}}$.
Each atom has charge = ${\text{ - 1}}$. So, ${\text{3}}$ atoms have charge = $ - 1 \times 3 = - 3$.
Total number of ${\text{O - atoms}}$ present = ${\text{4}}$. So, Formal charge of each ${\text{O - atom}}$
= $\dfrac{{{\text{Total charge}}}}{{{\text{Total atoms}}}}$
= $\dfrac{{ - 3}}{4}$
$ = - 0 \cdot 75$
Step (2): Calculation of ${\text{P - O}}$ bond order:
Total number of bonds in $P{O_4}^{3 - }$ = ${\text{5}}$, and Total number of resonating structures of $P{O_4}^{3 - }$ = ${\text{4}}$
Now, Bond order = $\dfrac{{{\text{Total number of bonds in the molecule}}}}{{{\text{Total number of resonating structures}}}}$
= $\dfrac{5}{4}$
= $1 \cdot 25$
So, the formal charge on each ${\text{O - atom}}$ is $ - 0 \cdot 75$, and ${\text{P - O}}$ bond order is $1 \cdot 25$.
So, the correct answer is Option C .
Note:
In the formula of Formal charge, do not write the number of pairs of electrons, instead write the total number of electrons in each pair. Also, the bond order is inversely proportional to the bond length between the atoms in consideration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




