
In phenol carbon atom attached to –OH group undergoes:
A. $s{p^3}$ hybridization
B. $sp$ hybridization
C. $s{p^2}$ hybridization
D. No hybridization
Answer
579.6k+ views
Hint: We have to know that the formation of a similar number of orbitals containing the same properties of different kinds of orbitals (s&p) of carbon atom is known as hybridization and orbitals produced are called hybridized orbitals.
Complete step by step answer:
We have to know that sigma bonds are formed by hybrid orbitals of carbon form and those orbitals which do not participate in hybridization to form pi (p) bonds.
A sigma bond is always a single bond. One sigma and one pi bond are found in double bonds. One sigma and two pi bonds are found in triple bonds.
In $ - C - C$ bond, the type of hybridization on the carbon atom is $s{p^3}$.
In $ > C = C < $ bond, the type of hybridization on the carbon atom is $s{p^2}$.
In $ - C \equiv C - $ bond, the type of hybridization on the carbon atom is $sp$.
We can draw the structure of phenol as,
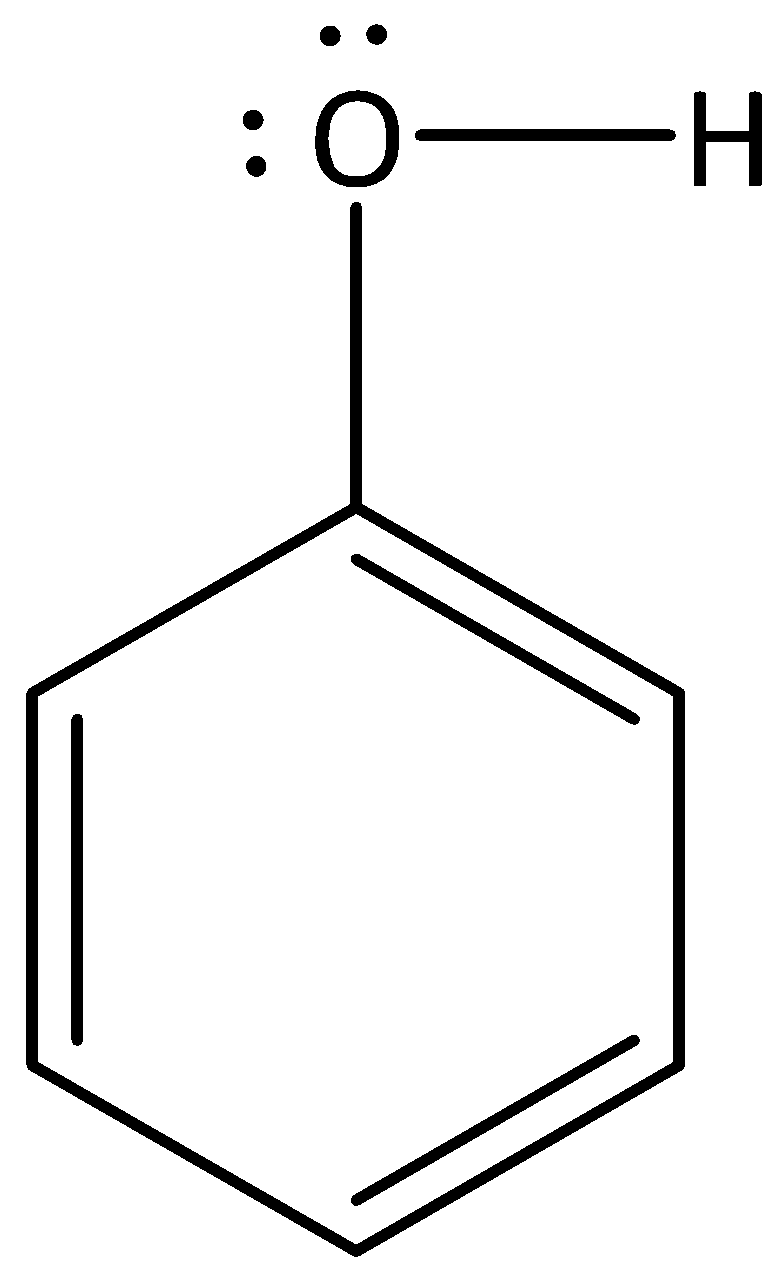
We have to know that in phenol, the carbon to which the hydroxyl group is attached belongs to $s{p^2}$ hybridization. The increased s-character of the carbon is used to give a $C - O$ bond and makes it a more electron withdrawing group. So, the conjugation with an aromatic ring will make the $s{p^2}$ hybridized atom of oxygen more likely.
So in phenol, the carbon atom bonded to the hydroxyl group is attached with three sigma bonds and one pi bond. So, the hybridization is $s{p^2}$.
So option (C) is correct.
Note:
If a compound contains single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkanes come under $s{p^3}$ hybridization. Molecules that have $s{p^3}$ hybridization will have tetrahedral geometry.
If a compound contains double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes come under $s{p^2}$ hybridization. Molecules that have $s{p^2}$ hybridization will have trigonal planar geometry.
If a compound contains triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes come under $sp$ hybridization.
Complete step by step answer:
We have to know that sigma bonds are formed by hybrid orbitals of carbon form and those orbitals which do not participate in hybridization to form pi (p) bonds.
A sigma bond is always a single bond. One sigma and one pi bond are found in double bonds. One sigma and two pi bonds are found in triple bonds.
In $ - C - C$ bond, the type of hybridization on the carbon atom is $s{p^3}$.
In $ > C = C < $ bond, the type of hybridization on the carbon atom is $s{p^2}$.
In $ - C \equiv C - $ bond, the type of hybridization on the carbon atom is $sp$.
We can draw the structure of phenol as,

We have to know that in phenol, the carbon to which the hydroxyl group is attached belongs to $s{p^2}$ hybridization. The increased s-character of the carbon is used to give a $C - O$ bond and makes it a more electron withdrawing group. So, the conjugation with an aromatic ring will make the $s{p^2}$ hybridized atom of oxygen more likely.
So in phenol, the carbon atom bonded to the hydroxyl group is attached with three sigma bonds and one pi bond. So, the hybridization is $s{p^2}$.
So option (C) is correct.
Note:
If a compound contains single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkanes come under $s{p^3}$ hybridization. Molecules that have $s{p^3}$ hybridization will have tetrahedral geometry.
If a compound contains double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes come under $s{p^2}$ hybridization. Molecules that have $s{p^2}$ hybridization will have trigonal planar geometry.
If a compound contains triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes come under $sp$ hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




