
In order to wash clothes with water containing dissolved calcium hydrogen carbonate, which cleaning agent will you prefer and why? Soaps or synthetic detergents? Give one advantage of soaps- over synthetic detergents.
Answer
513.6k+ views
Hint: The calcium hydrogen carbonate is also known as calcium bicarbonate having the chemical formula \[Ca{(HC{O_3})_2}\]. And it exists in the form of aqueous solution which contains calcium, bicarbonate, and carbonate ions and it is dissolved in carbon dioxide. The synthetic reagent is a non-soap cleanser and it mainly reduces the surface tension.
Complete answer:
If the water contains high amounts of minerals like calcium and magnesium, or calcium hydrogen carbonate, that type of water is known as hard water. Hence, the water containing dissolved calcium hydrogen carbonate is hard water. And to wash clothes with this hard water, will prefer the detergents. Because, the detergent contains the calcium salt and these calcium salts are soluble with hard water. Hence, we can easily remove the dirt on the clothes by using these detergents.
But the calcium salts present in the soaps are not soluble in hard water and the dirt will not be removed properly by using soap. Therefore, detergents are better to wash clothes with water containing dissolved calcium hydrogen carbonate. And the synthetic detergent can be drawn as,
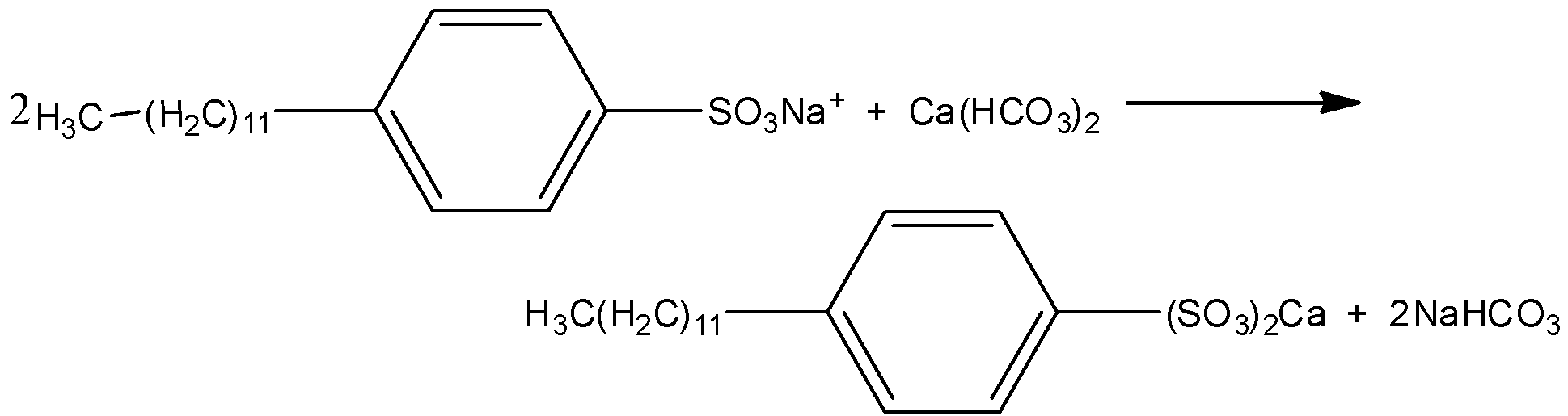
The soap has some disadvantages over synthetic detergents. And that is, the soap is biodegradable. Because it contains only fats and oils and it can easily break into simple molecules. But the detergents are made of chains of branched hydrocarbons and it cannot easily break down. Hence, detergents are not biodegradable and it causes pollution.
Note:
The detergents are used to wash clothes with water containing dissolved calcium hydrogen carbonate. If the water contains minerals like calcium carbonate, calcium, magnesium etc, and that type of water is known as hard water. And the detergents can easily work with these hard water which removes the dirt properly from the cloth.
Complete answer:
If the water contains high amounts of minerals like calcium and magnesium, or calcium hydrogen carbonate, that type of water is known as hard water. Hence, the water containing dissolved calcium hydrogen carbonate is hard water. And to wash clothes with this hard water, will prefer the detergents. Because, the detergent contains the calcium salt and these calcium salts are soluble with hard water. Hence, we can easily remove the dirt on the clothes by using these detergents.
But the calcium salts present in the soaps are not soluble in hard water and the dirt will not be removed properly by using soap. Therefore, detergents are better to wash clothes with water containing dissolved calcium hydrogen carbonate. And the synthetic detergent can be drawn as,

The soap has some disadvantages over synthetic detergents. And that is, the soap is biodegradable. Because it contains only fats and oils and it can easily break into simple molecules. But the detergents are made of chains of branched hydrocarbons and it cannot easily break down. Hence, detergents are not biodegradable and it causes pollution.
Note:
The detergents are used to wash clothes with water containing dissolved calcium hydrogen carbonate. If the water contains minerals like calcium carbonate, calcium, magnesium etc, and that type of water is known as hard water. And the detergents can easily work with these hard water which removes the dirt properly from the cloth.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




