
In iodide of Millon’s base formed by the reaction of Nessler’s reagent with $N{{H}_{3}}$, the coordination number of $Hg$ will be:
Answer
570.9k+ views
Hint: Nessler’s reagent is alkaline solution of potassium tetraiodomercurate (II) or ${{K}_{2}}Hg{{I}_{4}}$
The formation of iodide of Millon’s base is one of the qualitative tests for ammonium ions in the laboratory.
Complete step by step answer:
Before calculating the coordination number of $Hg$ in the iodide of Millon’s base, we should know about the formulae or the structural formulae of the complex formed, the ligand involved and the reaction through which it is formed.
So I have a basic idea of the reagents and we know that the Nessler’s reagent is the alkaline solution of ${{K}_{2}}Hg{{I}_{4}}$. Alkali like potassium iodide or potassium hydroxide is used.
Nessler’s reagent is prepared by mixing mercuric iodide or mercuric chloride with potassium iodide and this mixture is made basic using an alkali like NaOH or KOH.
Nessler’s reagent on reaction with the solution containing the ammonium ions ($N{{H}_{4}}^{+}$) gives a brown precipitate whereas if the reaction is done between the ammonia vapors and the Nessler’s reagent then brown fumes are produced. The brown precipitate formed is called the iodide of Millon’s base.
This reaction is used for the qualitative analysis and for the determination of $N{{H}_{4}}^{+}$ ions in the unknown solutions.
The reaction involving the formation of iodide of Millon’s base is as follows:
$2{{K}_{2}}\left[ Hg{{I}_{4}} \right]+N{{H}_{3}}+3KOH\to \left[ OH{{g}_{2}}.N{{H}_{2}} \right]I+7KI+2{{H}_{2}}O$
Now we have to find the coordination number of iodide of Millon’s base. We know that the coordination number is the number of ligands attached to the central metal atom. So more understanding let's draw the structure of iodide of Millon’s base,
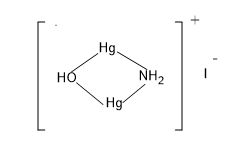
So from the figure it’s clear that two ligands are associated with the central metal atom.
Hence the coordination number of $Hg$ in iodide of Millon’s base is $2$.
Note: The reagent is very sensitive towards the $N{{H}_{4}}^{+}$ ions as even for small concentration of $N{{H}_{4}}^{+}$ ions brown coloration is produced and brown precipitate is produced if the concentration of $N{{H}_{4}}^{+}$ ions are large. So we have to add quite a large amount of ammonium salts to obtain the brown precipitate of iodide of Millon’s base.
The formation of iodide of Millon’s base is one of the qualitative tests for ammonium ions in the laboratory.
Complete step by step answer:
Before calculating the coordination number of $Hg$ in the iodide of Millon’s base, we should know about the formulae or the structural formulae of the complex formed, the ligand involved and the reaction through which it is formed.
So I have a basic idea of the reagents and we know that the Nessler’s reagent is the alkaline solution of ${{K}_{2}}Hg{{I}_{4}}$. Alkali like potassium iodide or potassium hydroxide is used.
Nessler’s reagent is prepared by mixing mercuric iodide or mercuric chloride with potassium iodide and this mixture is made basic using an alkali like NaOH or KOH.
Nessler’s reagent on reaction with the solution containing the ammonium ions ($N{{H}_{4}}^{+}$) gives a brown precipitate whereas if the reaction is done between the ammonia vapors and the Nessler’s reagent then brown fumes are produced. The brown precipitate formed is called the iodide of Millon’s base.
This reaction is used for the qualitative analysis and for the determination of $N{{H}_{4}}^{+}$ ions in the unknown solutions.
The reaction involving the formation of iodide of Millon’s base is as follows:
$2{{K}_{2}}\left[ Hg{{I}_{4}} \right]+N{{H}_{3}}+3KOH\to \left[ OH{{g}_{2}}.N{{H}_{2}} \right]I+7KI+2{{H}_{2}}O$
Now we have to find the coordination number of iodide of Millon’s base. We know that the coordination number is the number of ligands attached to the central metal atom. So more understanding let's draw the structure of iodide of Millon’s base,

So from the figure it’s clear that two ligands are associated with the central metal atom.
Hence the coordination number of $Hg$ in iodide of Millon’s base is $2$.
Note: The reagent is very sensitive towards the $N{{H}_{4}}^{+}$ ions as even for small concentration of $N{{H}_{4}}^{+}$ ions brown coloration is produced and brown precipitate is produced if the concentration of $N{{H}_{4}}^{+}$ ions are large. So we have to add quite a large amount of ammonium salts to obtain the brown precipitate of iodide of Millon’s base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




