
In fcc structure, octahedral sites are present at:
A). Edge centres
B). Face centres
C). Body centers
D). Corners
Answer
584.4k+ views
Hint: The octahedral voids are located at the body centres and at the centres of the 12 edges of the cube. Number of octahedral voids per unit cell in a cubic close packing is 4.
Complete step-by-step answer:
-Let us consider the unit cell of cubic close packing lattice structure.
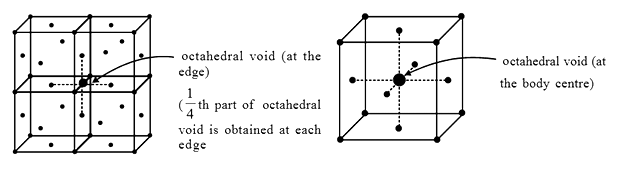
-The body centre of the cube is not occupied by any atom but its surrounded by six atoms at the face centres.
-If the atoms at the face centres are joined, a regular octahedron is generated.
-Thus, an octahedral void is located at the body center of the unit cell of ccp lattice.
-In addition to the octahedral void at the body centre there are 12 octahedral voids at the centres of the 12 edges of the cube.
-Each octahedral void on the edge centre is being shared by four unit cells.
-Thus, in cubic close packing the number of octahedral voids per unit cell can be calculated as under:
Number of octahedral voids per unit cell in a cubic close packing
= 1 (centre of the cubic) + 12 (at edge centres) x $1 / 4$
= 1 + 3 = 4
Clearly the answers are A and C.
Note: Do not confuse with the tetrahedral voids. There is one such void in the tetrahedron of ccp lattice. There are a total of eight tetrahedral voids in the unit cell of ccp structure. They are located on the body centres of the cube structure.
Complete step-by-step answer:
-Let us consider the unit cell of cubic close packing lattice structure.

-The body centre of the cube is not occupied by any atom but its surrounded by six atoms at the face centres.
-If the atoms at the face centres are joined, a regular octahedron is generated.
-Thus, an octahedral void is located at the body center of the unit cell of ccp lattice.
-In addition to the octahedral void at the body centre there are 12 octahedral voids at the centres of the 12 edges of the cube.
-Each octahedral void on the edge centre is being shared by four unit cells.
-Thus, in cubic close packing the number of octahedral voids per unit cell can be calculated as under:
Number of octahedral voids per unit cell in a cubic close packing
= 1 (centre of the cubic) + 12 (at edge centres) x $1 / 4$
= 1 + 3 = 4
Clearly the answers are A and C.
Note: Do not confuse with the tetrahedral voids. There is one such void in the tetrahedron of ccp lattice. There are a total of eight tetrahedral voids in the unit cell of ccp structure. They are located on the body centres of the cube structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




