
In diammonium hydrogen phosphate, ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$, the percentage of:
(This question has multiple correct options.)
(A) ${{P}_{2}}{{O}_{5}}$ is 53.78%
(B) $N{{H}_{3}}$ is 25.76%
(C) P is maximum
(D) N is minimum
Answer
531k+ views
Hint: To solve this question we first need to determine the molecular mass of diammonium hydrogen phosphate, ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$. The molecular mass of one mole of a compound can be calculated by taking the sum of atomic masses of all the elements present in the molecule.
Complete answer:
Now, we know that the atomic mass of
N = 14.01 u
H = 1.010 u
P = 30.97 u
O = 16.00 u
Diammonium hydrogen phosphate, ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$, can also be written as ${{H}_{9}}{{N}_{2}}{{O}_{4}}P$.
So, the molar mass of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) will be
\[\begin{align}
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=2\times {{M}_{N}}+9\times {{M}_{H}}+4\times {{M}_{O}}+{{M}_{P}} \\
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=2\times 14.01+9\times 1.01+4\times 16+30.97 \\
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=132.06\text{ g/mol} \\
\end{align}\]
Now, the formula to calculate the percentage composition of a constituent in a compound is given by:
\[%\text{ composition = }\dfrac{\text{mass of molecule in 1 mole of compound}}{\text{molar mass of compound}}\times 100\]
So, the percentage compositions of elements N, H, O, and P are as follows
\[\begin{align}
& (N)=\dfrac{2\times 14.01}{132.06}\times 100=21.21 \\
& (H)=\dfrac{9\times 1.01}{132.06}\times 100=6.87 \\
& (O)=\dfrac{4\times 16}{132.06}\times 100=48.46 \\
& (P)=\dfrac{30.97}{132.06}\times 100=23.45 \\
\end{align}\]
From the above calculations, we can see that the percentage compositions of elements in ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$ are
N = 21.21%
H = 6.870%
O = 48.46%
P = 23.45 %
Now, diammonium hydrogen phosphate is produced as follows
\[{{P}_{2}}{{O}_{5}}+4N{{H}_{3}}+3{{H}_{2}}O\to 2{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}\]
Hence, we can say that one mole of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) consists of a half mole of phosphorus pentoxide (${{P}_{2}}{{O}_{5}}$).
Also, it can be said that one mole of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) consists of two moles of ammonia ($N{{H}_{3}}$).
So, the percentage compositions of ${{P}_{2}}{{O}_{5}}$ and $N{{H}_{3}}$ will be
$\begin{align}
& ({{P}_{2}}{{O}_{5}})=\dfrac{0.5\times [2\times 30.97+5\times 16]}{132.06}\times 100=53.78 \\
& (N{{H}_{3}})=\dfrac{2\times [14.01+3\times 1.01]}{132.06}\times 100=25.76 \\
\end{align}$
From the above calculations, we can see that the percentage compositions of ${{P}_{2}}{{O}_{5}}$ and $N{{H}_{3}}$ in ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$ are
${{P}_{2}}{{O}_{5}}$ = 53.78%
$N{{H}_{3}}$ = 25.76%
So, the correct answers are option (A) and option (B).
Note:
It should be noted that upon dissociation at a temperature of $100{}^\circ C$ and dissociation pressure of 5mmHg, diammonium hydrogen phosphate dissociates as follows
\[{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}(s)\rightleftarrows N{{H}_{3}}(g)+(N{{H}_{4}}){{H}_{2}}P{{O}_{4}}(s)\]
The structure of diammonium hydrogen phosphate salt is as follows.
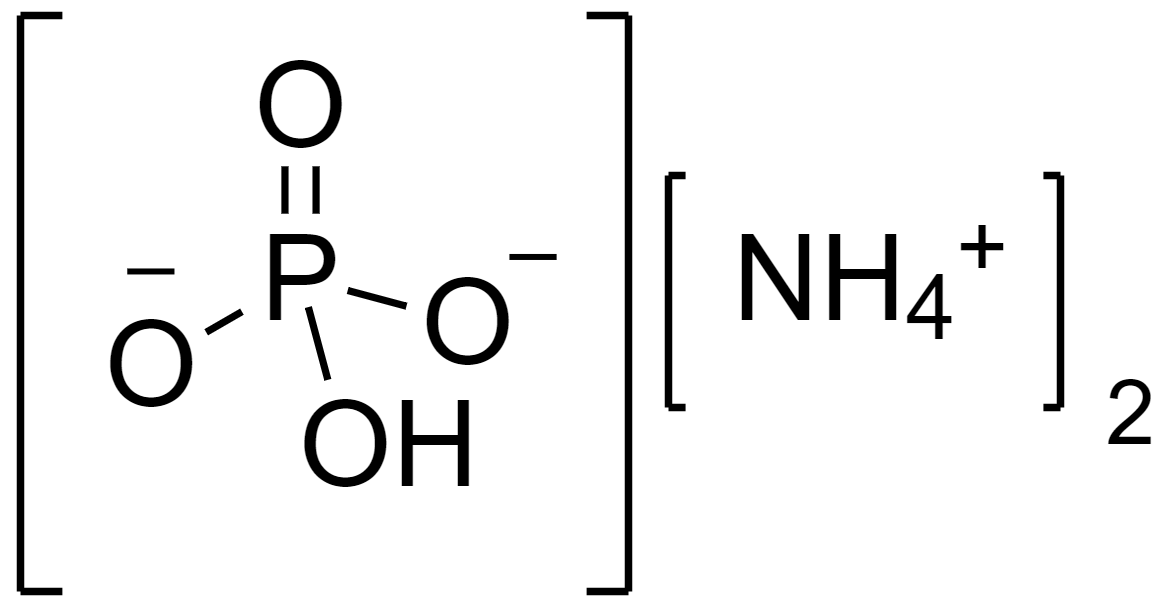
Complete answer:
Now, we know that the atomic mass of
N = 14.01 u
H = 1.010 u
P = 30.97 u
O = 16.00 u
Diammonium hydrogen phosphate, ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$, can also be written as ${{H}_{9}}{{N}_{2}}{{O}_{4}}P$.
So, the molar mass of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) will be
\[\begin{align}
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=2\times {{M}_{N}}+9\times {{M}_{H}}+4\times {{M}_{O}}+{{M}_{P}} \\
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=2\times 14.01+9\times 1.01+4\times 16+30.97 \\
& {{M}_{{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}}}=132.06\text{ g/mol} \\
\end{align}\]
Now, the formula to calculate the percentage composition of a constituent in a compound is given by:
\[%\text{ composition = }\dfrac{\text{mass of molecule in 1 mole of compound}}{\text{molar mass of compound}}\times 100\]
So, the percentage compositions of elements N, H, O, and P are as follows
\[\begin{align}
& (N)=\dfrac{2\times 14.01}{132.06}\times 100=21.21 \\
& (H)=\dfrac{9\times 1.01}{132.06}\times 100=6.87 \\
& (O)=\dfrac{4\times 16}{132.06}\times 100=48.46 \\
& (P)=\dfrac{30.97}{132.06}\times 100=23.45 \\
\end{align}\]
From the above calculations, we can see that the percentage compositions of elements in ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$ are
N = 21.21%
H = 6.870%
O = 48.46%
P = 23.45 %
Now, diammonium hydrogen phosphate is produced as follows
\[{{P}_{2}}{{O}_{5}}+4N{{H}_{3}}+3{{H}_{2}}O\to 2{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}\]
Hence, we can say that one mole of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) consists of a half mole of phosphorus pentoxide (${{P}_{2}}{{O}_{5}}$).
Also, it can be said that one mole of diammonium hydrogen phosphate (${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$) consists of two moles of ammonia ($N{{H}_{3}}$).
So, the percentage compositions of ${{P}_{2}}{{O}_{5}}$ and $N{{H}_{3}}$ will be
$\begin{align}
& ({{P}_{2}}{{O}_{5}})=\dfrac{0.5\times [2\times 30.97+5\times 16]}{132.06}\times 100=53.78 \\
& (N{{H}_{3}})=\dfrac{2\times [14.01+3\times 1.01]}{132.06}\times 100=25.76 \\
\end{align}$
From the above calculations, we can see that the percentage compositions of ${{P}_{2}}{{O}_{5}}$ and $N{{H}_{3}}$ in ${{(N{{H}_{4}})}_{2}}HP{{O}_{4}}$ are
${{P}_{2}}{{O}_{5}}$ = 53.78%
$N{{H}_{3}}$ = 25.76%
So, the correct answers are option (A) and option (B).
Note:
It should be noted that upon dissociation at a temperature of $100{}^\circ C$ and dissociation pressure of 5mmHg, diammonium hydrogen phosphate dissociates as follows
\[{{(N{{H}_{4}})}_{2}}HP{{O}_{4}}(s)\rightleftarrows N{{H}_{3}}(g)+(N{{H}_{4}}){{H}_{2}}P{{O}_{4}}(s)\]
The structure of diammonium hydrogen phosphate salt is as follows.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




