
In crossed cannizzaro reactions between formaldehyde and Benzaldehyde the alcohol formed is only benzyl alcohol and methanol. Give reason.
Answer
589.5k+ views
Hint: We know that if an aldehyde without any $\alpha $ -hydrogen is made to react with formaldehyde in presence of a base to give sodium formate and an alcohol, it is called as Cross Cannizzaro reaction.
Complete step by step answer:
Aldehydes with alpha hydrogen atoms undergo deprotonation due to the strongly alkaline conditions of the reaction, resulting in enolates and/or aldol reactions of those enolates where beta-hydroxy aldehydes or ketones are obtained. Therefore, it's not surprising that the reaction produces only \[50\%\] of the specified alcohol and acid at ideal conditions. This is often why the crossed Cannizzaro reaction is more commonly used. A sacrificial aldehyde is combined with a more valuable chemical and formaldehyde is employed as a reductant, oxidizing it to sodium formate. The specified alcohol is obtained from the reduction of the opposite aldehyde chemical. Since two different aldehydes are often completely converted into the specified product, the yield of the precious chemical is increased.
We can write the chemical equation for the Crossed Cannizzaro reaction between formaldehyde and Benzaldehyde as,
\[{C_6}{H_5} - CHO + \left( {Formaldehyde} \right)HCHO\xrightarrow{{NaOH}}HCOOH + {C_6}{H_5} - C{H_2} - OH\]
As there is no electron donating group on formaldehyde, the initial nucleophilic addition of hydroxide ion is faster on formaldehyde due to which formaldehyde oxidizes easily and forms formic acid. Thus, the alcohol formed is only benzyl alcohol and not methanol.
Note:
We must remember that the Cannizzaro reaction may be a reaction named after Stanislao Cannizzaro that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield an acid and a primary alcohol.
Now we can discuss about the example of Cannizzaro reaction as,
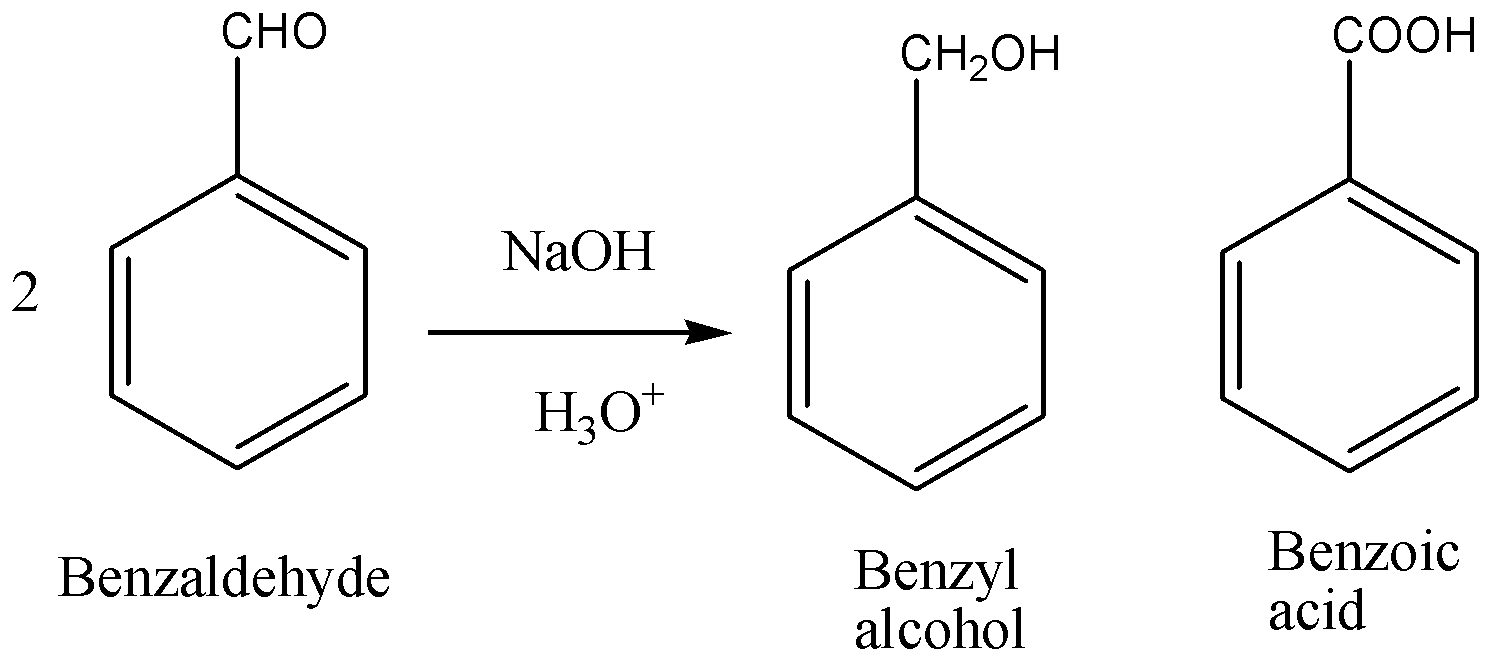
When two molecules of benzaldehyde are treated with $NaOH$ to give benzyl alcohol and benzoic acid.
We can write the chemical equation for the above reaction as,
Complete step by step answer:
Aldehydes with alpha hydrogen atoms undergo deprotonation due to the strongly alkaline conditions of the reaction, resulting in enolates and/or aldol reactions of those enolates where beta-hydroxy aldehydes or ketones are obtained. Therefore, it's not surprising that the reaction produces only \[50\%\] of the specified alcohol and acid at ideal conditions. This is often why the crossed Cannizzaro reaction is more commonly used. A sacrificial aldehyde is combined with a more valuable chemical and formaldehyde is employed as a reductant, oxidizing it to sodium formate. The specified alcohol is obtained from the reduction of the opposite aldehyde chemical. Since two different aldehydes are often completely converted into the specified product, the yield of the precious chemical is increased.
We can write the chemical equation for the Crossed Cannizzaro reaction between formaldehyde and Benzaldehyde as,
\[{C_6}{H_5} - CHO + \left( {Formaldehyde} \right)HCHO\xrightarrow{{NaOH}}HCOOH + {C_6}{H_5} - C{H_2} - OH\]
As there is no electron donating group on formaldehyde, the initial nucleophilic addition of hydroxide ion is faster on formaldehyde due to which formaldehyde oxidizes easily and forms formic acid. Thus, the alcohol formed is only benzyl alcohol and not methanol.
Note:
We must remember that the Cannizzaro reaction may be a reaction named after Stanislao Cannizzaro that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield an acid and a primary alcohol.
Now we can discuss about the example of Cannizzaro reaction as,
When two molecules of benzaldehyde are treated with $NaOH$ to give benzyl alcohol and benzoic acid.
We can write the chemical equation for the above reaction as,

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




