
In $CO$ the hybridization state of $C$ is:
Answer
583.5k+ views
Hint:The orbital hybridization includes the concept of mixing of the atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the individual atomic orbitals) which are suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Complete step by step answer:
Hybrid orbitals are very useful in the explanation of geometry of the molecules and atomic bonding properties and are symmetrically oriented in space. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridization are in fact not related to the VSEPR model. Carbon and oxygen together have a total of 10 electrons in the valence shell. Following the octet rule for both carbon and oxygen, the two atoms form a triple bond, with six shared electrons in three bonding molecular orbitals, rather than the usual double bond found in organic carbonyl compounds. The electronic configuration of carbon is $1{s^2}2{s^2}2{p^2}$ and that of oxygen is $1{s^2}2{s^2}2{p^4}$ . This means that carbon has two $p - $ orbitals to bond with oxygen and produce two $sp$ hybridized orbitals. The carbon atom still has a vacant orbital and thus, receives a lone pair of electrons from the oxygen atom through back bonding and forms a coordinate bond. The carbon monoxide is a linear molecule with bond angle of ${180^o}$ . The structure of carbon monoxide is as follows:
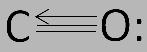
Thus, the hybridization of carbon atoms in $CO$ is $sp$ .
Note:Since four of the shared electrons come from the oxygen atom and only two from carbon, one bonding orbital is occupied by two electrons from oxygen, forming a dative or dipolar bond. This causes a \[C \to O\] polarization of the molecule. The other two bonding orbitals are each occupied by one electron from carbon and one from oxygen, forming polar covalent bonds with a reverse \[C \to O\] polarization, since oxygen is more electronegative than carbon.
Complete step by step answer:
Hybrid orbitals are very useful in the explanation of geometry of the molecules and atomic bonding properties and are symmetrically oriented in space. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridization are in fact not related to the VSEPR model. Carbon and oxygen together have a total of 10 electrons in the valence shell. Following the octet rule for both carbon and oxygen, the two atoms form a triple bond, with six shared electrons in three bonding molecular orbitals, rather than the usual double bond found in organic carbonyl compounds. The electronic configuration of carbon is $1{s^2}2{s^2}2{p^2}$ and that of oxygen is $1{s^2}2{s^2}2{p^4}$ . This means that carbon has two $p - $ orbitals to bond with oxygen and produce two $sp$ hybridized orbitals. The carbon atom still has a vacant orbital and thus, receives a lone pair of electrons from the oxygen atom through back bonding and forms a coordinate bond. The carbon monoxide is a linear molecule with bond angle of ${180^o}$ . The structure of carbon monoxide is as follows:
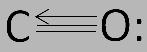
Thus, the hybridization of carbon atoms in $CO$ is $sp$ .
Note:Since four of the shared electrons come from the oxygen atom and only two from carbon, one bonding orbital is occupied by two electrons from oxygen, forming a dative or dipolar bond. This causes a \[C \to O\] polarization of the molecule. The other two bonding orbitals are each occupied by one electron from carbon and one from oxygen, forming polar covalent bonds with a reverse \[C \to O\] polarization, since oxygen is more electronegative than carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




