
In benzene what is the hybridization of each carbon atom?
A.\[s{p^2}\]
B.\[s{p^3}\]
C.\[s{p^3}d\]
D.\[sp\]
Answer
504.3k+ views
Hint: Benzene is an aromatic carbon compound containing alternative double bonds between the carbon atoms. Each carbon is connected to two other carbon atoms of the ring and one hydrogen atom that lies outside the ring.
Complete answer:
The concept of hybridization comes from the idea of mixing pure atomic orbitals into hybrid orbitals to have efficient overlaps while bonding.
Carbon is a tetravalent atom and forms four covalent bonds in all its compounds. This does not mean that it must form four individual bonds with four different atoms, it has a tendency to form double or triple bonds with a single atom to satisfy its valency. The double or triple bonded system may contain another carbon atom or an oxygen or nitrogen atom.
Aromatic compounds formed by carbon consist of continuous delocalization of electrons due to the presence of alternate double bonds. The structure of benzene is such that each carbon atom is connected to two adjacent carbon atoms of the ring with one single bond and one double bond. A hydrogen atom gets bonded to each carbon through a single bond. Thus each carbon is connected to three other atoms.
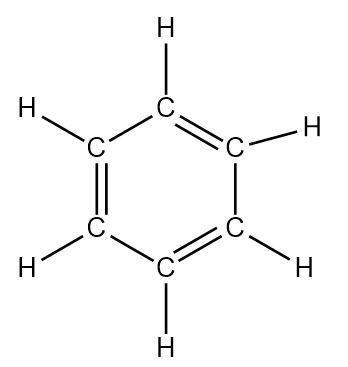
In order to bond with three atoms, the carbon must mix three of its orbitals keeping one \[p\] orbital free for forming double bonds through sidewise overlap (\[\pi - bonds\] ). Thus one \[s\] and two \[p\] orbitals must hybridize to give three equivalent \[s{p^2}\] orbitals.
Hence, the correct option is (A), all the carbon atoms in benzene are \[s{p^2}\] hybridized.
Note:
The hybridization is important to obtain mixed orbitals that have the same sizes and energy. The hybridized orbitals are most suitable for overlapping as they contain the directional character as well as suitable energy and larger lobes for overlapping, resulting in more efficient bonds.
Complete answer:
The concept of hybridization comes from the idea of mixing pure atomic orbitals into hybrid orbitals to have efficient overlaps while bonding.
Carbon is a tetravalent atom and forms four covalent bonds in all its compounds. This does not mean that it must form four individual bonds with four different atoms, it has a tendency to form double or triple bonds with a single atom to satisfy its valency. The double or triple bonded system may contain another carbon atom or an oxygen or nitrogen atom.
Aromatic compounds formed by carbon consist of continuous delocalization of electrons due to the presence of alternate double bonds. The structure of benzene is such that each carbon atom is connected to two adjacent carbon atoms of the ring with one single bond and one double bond. A hydrogen atom gets bonded to each carbon through a single bond. Thus each carbon is connected to three other atoms.

In order to bond with three atoms, the carbon must mix three of its orbitals keeping one \[p\] orbital free for forming double bonds through sidewise overlap (\[\pi - bonds\] ). Thus one \[s\] and two \[p\] orbitals must hybridize to give three equivalent \[s{p^2}\] orbitals.
Hence, the correct option is (A), all the carbon atoms in benzene are \[s{p^2}\] hybridized.
Note:
The hybridization is important to obtain mixed orbitals that have the same sizes and energy. The hybridized orbitals are most suitable for overlapping as they contain the directional character as well as suitable energy and larger lobes for overlapping, resulting in more efficient bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




