
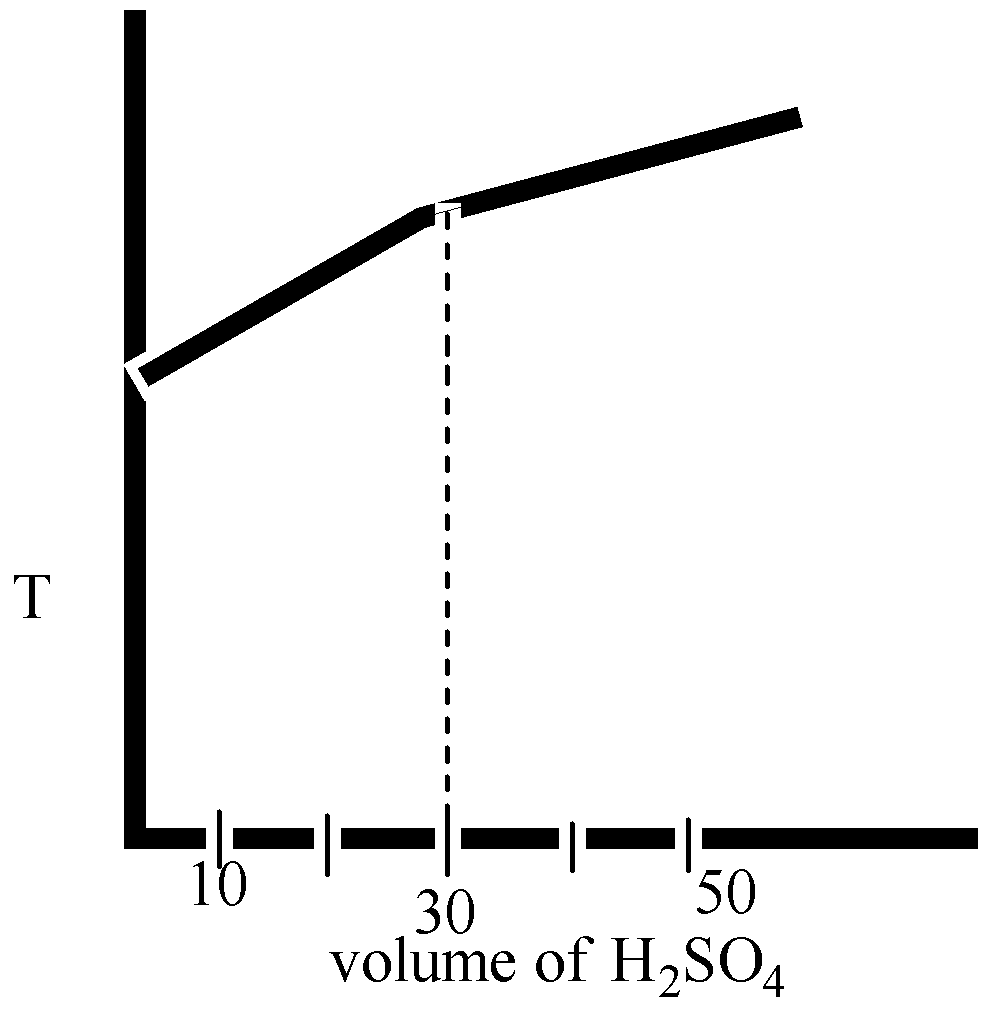
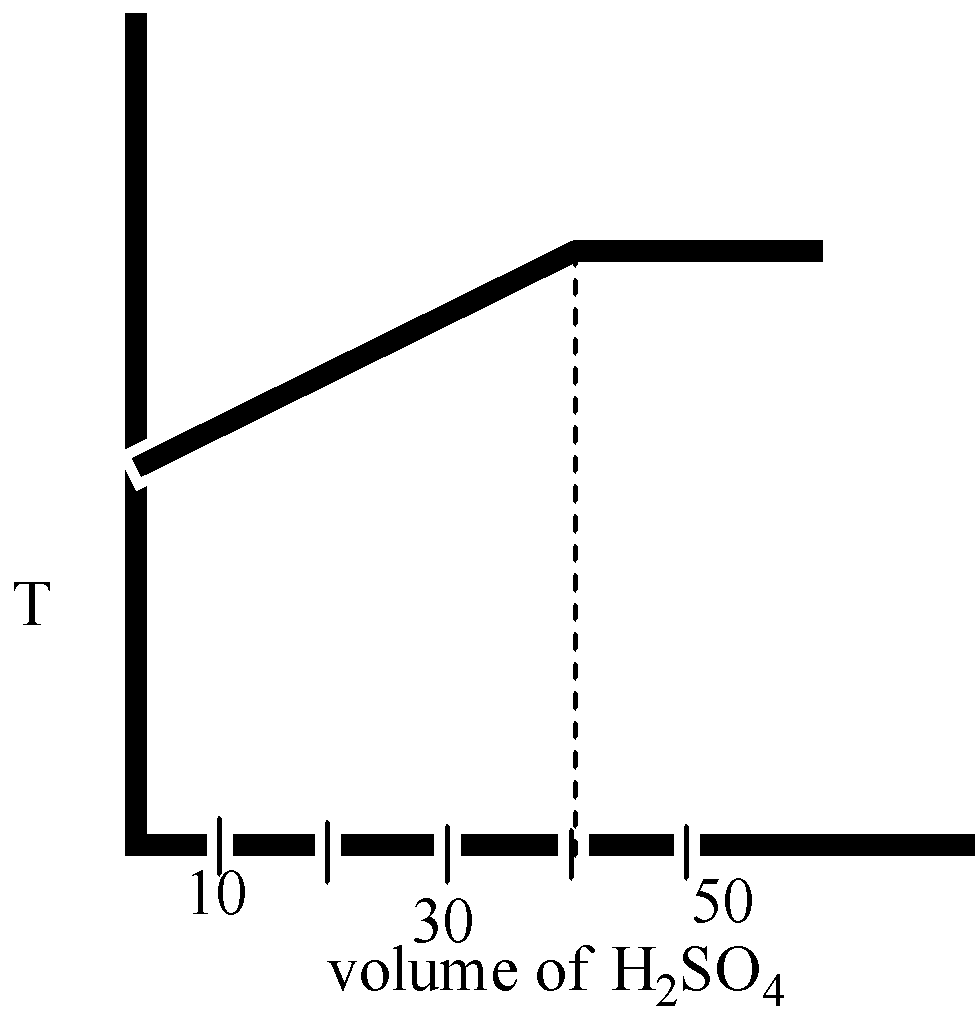
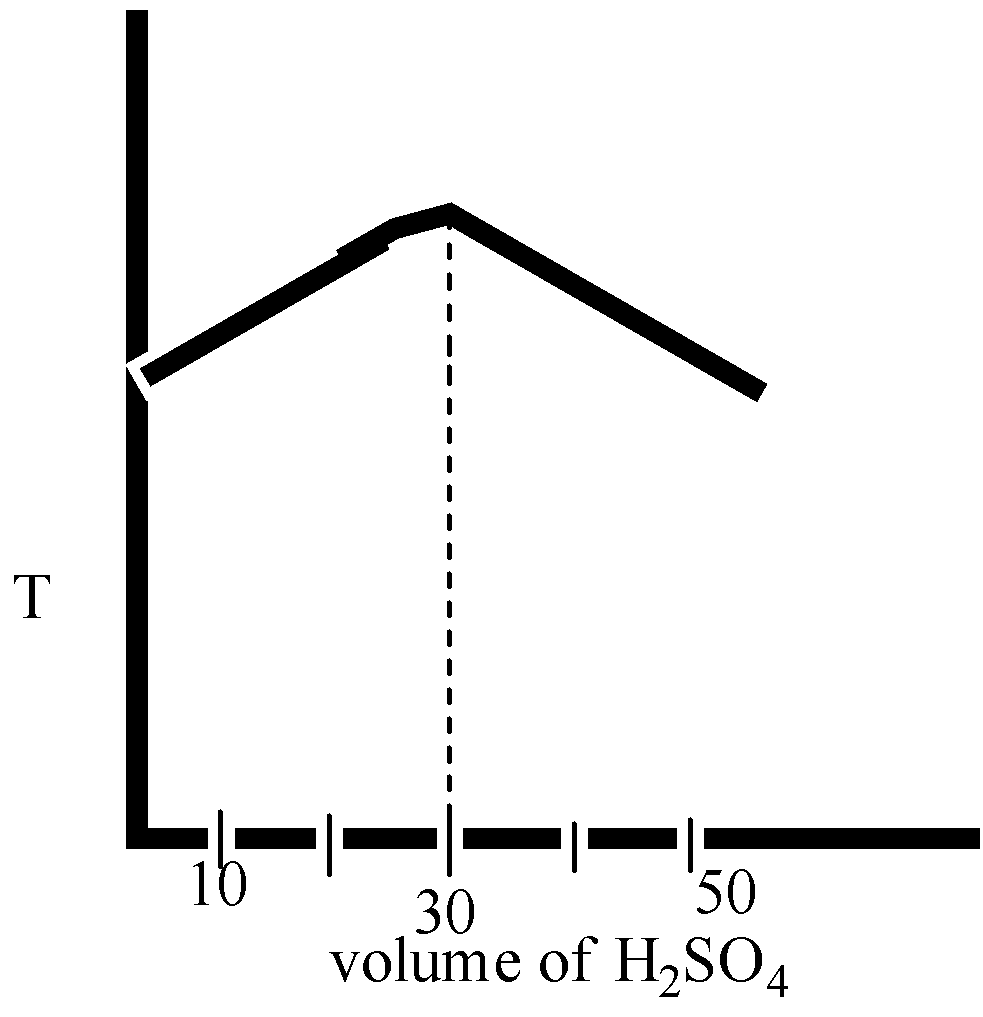
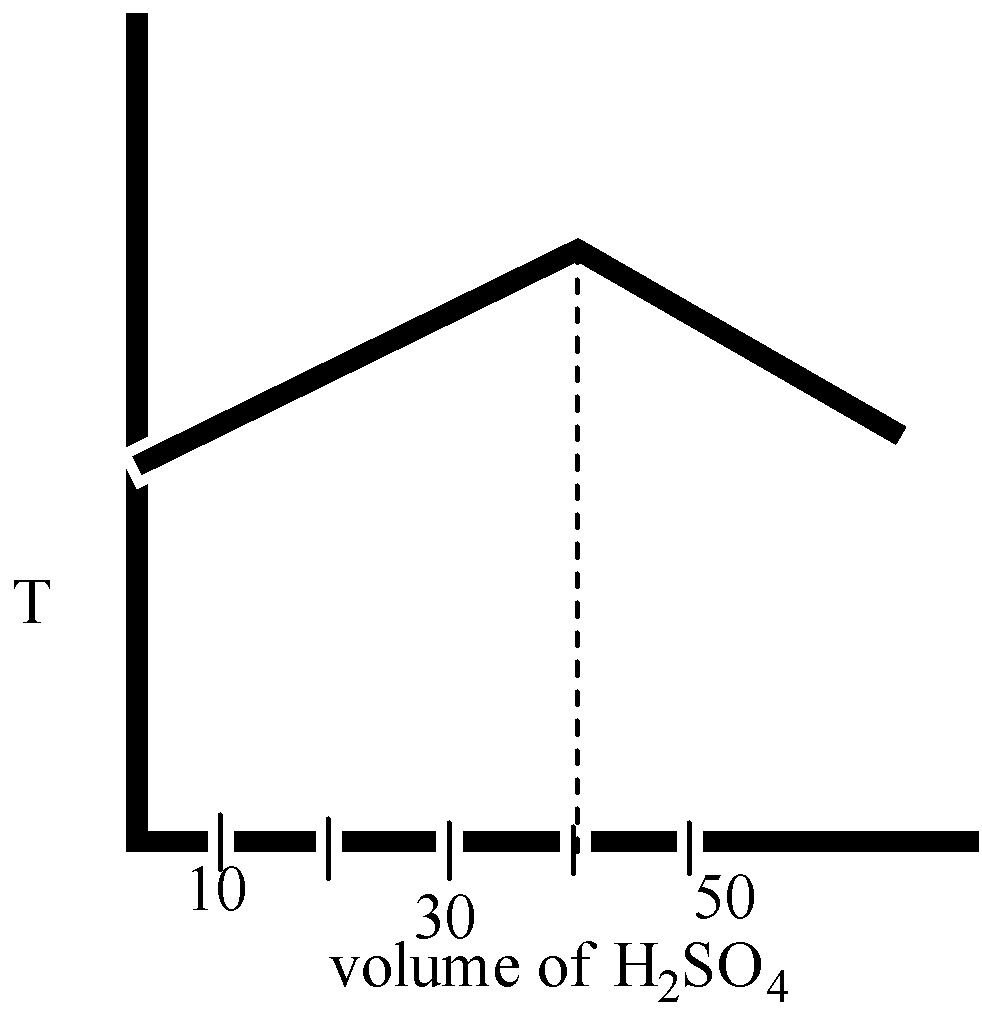
In an experiment to determine the enthalpy of neutralization of sodium hydroxide with sulphuric acid, ${{50c}}{{{m}}^{{3}}}$ of 0.4 M sodium hydroxide were titrated thermometrically with 0.25 M sulphuric acid. Which of the following plots gives the correct representation.
(A)
(B)
(C)
(D)




Answer
565.5k+ views
Hint:Enthalpy of neutralization is the heat change produced when acid is reacted with alkali under standard conditions. The reaction of an acid and a base gives water and salt. Neutralization reactions are exothermic and have a negative value. We know that sulphuric acid is a strong acid and sodium hydroxide is a strong base. $\left[ {{{molarity = }}\dfrac{{{{no. of moles}}}}{{{{volume}}}}} \right]$
Complete step by step answer:
Here, sodium hydroxide is titrated with sulphuric acid.
Let us take sodium hydroxide in the burette for titration. Drop by drop, sodium hydroxide is added to the sulphuric acid in the conical flask.
Sulphuric acid gets dissociated easily and the fast-moving ${{{H}}^{{ + }}}$ ions are replaced by the slow-moving ${{N}}{{{a}}^{{ + }}}$ ions. At one point the acid and the alkali get neutralized.
The volume of sulphuric acid is not known to us whereas the concentration and volume of sodium hydroxide are known.
Therefore the number of moles of NaOH reacting is ${{50c}}{{{m}}^{{3}}}{{ \times 0}}{{.4 M}}$
The actual concentration of NaOH is 20 moles.
We need to know what volume of sulphuric acid reacts with 50 mL of 0.4 M of sodium hydroxide.
${{2NaOH + }}{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}{{ }} \to {{N}}{{{a}}_{{2}}}{{S}}{{{O}}_{{4}}}{{ + 2}}{{{H}}_{{2}}}{{O}} \to {{eq 1}}$
Thus 20 moles of NaOH are reacting.
From the balanced chemical equation, we can say that 2 moles of NaOH are reacting with 1 mole of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ in the ratio ${{2:1}}$. So the sulphuric acid has half the number of moles of NaOH.
So we can say that 10 moles of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ are reacting ( 20 moles of NaOH reacted with x moles of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ in the ratio ${{2:1}}$ .
Now let us find the volume of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ which can be found from equation 1 that
${{Volume = }}\dfrac{{{{Moles}}}}{{{{Concentration}}}}$
${{volume = }}\dfrac{{{{10}}}}{{{{0}}{{.25}}}}$
The volume of sulphuric acid reacting is thus 40mL at the neutralization point.
That is the point at which neutralization is complete.
Here in option B and option D, the volume ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ is 40 mL.
We know that during neutralization, heat is evolved. During neutralization, the ${{{H}}^{{ + }}}$ ion from acid and ${{O}}{{{H}}^{{ - }}}$ base reacts to give a large amount of heat showing an increase in temperature. After neutralization, the ${{{H}}^{{ + }}}$ ions will be replaced by slow-moving ions and salt is formed. Thus, no more heat formation takes place.
Therefore, we can say that the temperature is decreased after the neutralization point.
The correct option is ( D).
Note:
If neutralization is done between a strong acid and weak base, more amount of weak base is used to neutralize the strong acid if both the concentrations are equal. Similarly, if the neutralization is done between a weak acid and a strong base at the same concentration, more volume of a weak base is required to neutralize the reaction.
Complete step by step answer:
Here, sodium hydroxide is titrated with sulphuric acid.
Let us take sodium hydroxide in the burette for titration. Drop by drop, sodium hydroxide is added to the sulphuric acid in the conical flask.
Sulphuric acid gets dissociated easily and the fast-moving ${{{H}}^{{ + }}}$ ions are replaced by the slow-moving ${{N}}{{{a}}^{{ + }}}$ ions. At one point the acid and the alkali get neutralized.
The volume of sulphuric acid is not known to us whereas the concentration and volume of sodium hydroxide are known.
Therefore the number of moles of NaOH reacting is ${{50c}}{{{m}}^{{3}}}{{ \times 0}}{{.4 M}}$
The actual concentration of NaOH is 20 moles.
We need to know what volume of sulphuric acid reacts with 50 mL of 0.4 M of sodium hydroxide.
${{2NaOH + }}{{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}{{ }} \to {{N}}{{{a}}_{{2}}}{{S}}{{{O}}_{{4}}}{{ + 2}}{{{H}}_{{2}}}{{O}} \to {{eq 1}}$
Thus 20 moles of NaOH are reacting.
From the balanced chemical equation, we can say that 2 moles of NaOH are reacting with 1 mole of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ in the ratio ${{2:1}}$. So the sulphuric acid has half the number of moles of NaOH.
So we can say that 10 moles of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ are reacting ( 20 moles of NaOH reacted with x moles of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ in the ratio ${{2:1}}$ .
Now let us find the volume of ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ which can be found from equation 1 that
${{Volume = }}\dfrac{{{{Moles}}}}{{{{Concentration}}}}$
${{volume = }}\dfrac{{{{10}}}}{{{{0}}{{.25}}}}$
The volume of sulphuric acid reacting is thus 40mL at the neutralization point.
That is the point at which neutralization is complete.
Here in option B and option D, the volume ${{{H}}_{{2}}}{{S}}{{{O}}_{{4}}}$ is 40 mL.
We know that during neutralization, heat is evolved. During neutralization, the ${{{H}}^{{ + }}}$ ion from acid and ${{O}}{{{H}}^{{ - }}}$ base reacts to give a large amount of heat showing an increase in temperature. After neutralization, the ${{{H}}^{{ + }}}$ ions will be replaced by slow-moving ions and salt is formed. Thus, no more heat formation takes place.
Therefore, we can say that the temperature is decreased after the neutralization point.
The correct option is ( D).
Note:
If neutralization is done between a strong acid and weak base, more amount of weak base is used to neutralize the strong acid if both the concentrations are equal. Similarly, if the neutralization is done between a weak acid and a strong base at the same concentration, more volume of a weak base is required to neutralize the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




